Methemoglobinemia is a hemoglobinopathy caused by high levels of methemoglobin that result from oxidation of ferrrous (Fe2+) to the ferric state (Fe3+) in hemoglogin. Methemoglobin cannot carry oxygen, leading to tissue hypoxia. Surgical patients rarely have methemoglobinemia, and most cases are mild and require no treatment. However, perioperative methemoglobinemia is often overlooked as a cause of low oxygen saturation, and is often mistaken for other pulmonary disorders such as atelectasis, pulmonary edema, pulmonary thromboembolism, or inadequate anesthetic care such as esophageal intubation. We report our experience of a dapsone-induced methemoglobinemia detected during general anesthesia of a 68-year-old female patient who underwent gastrectomy for advanced gastric cancer with hemorrhage and perforation.
Case Report
A 68-year-old female patient with advanced gastric cancer presented for a gastrectomy. She was under drug treatment for diabetes and hypertension. Preoperative complete blood count showed hemoglobin at 8.9 g/dl and hematocrit at 26.5%. Arterial blood gas analysis was not performed. The electrocardiography, chest x-ray, and pulmonary function test showed no abnormal findings.
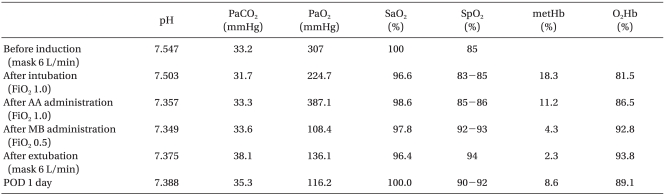
After arriving at the OR, the patient monitoring system (Polymount Philips, USA) was used to monitor the blood pressure, heart rate, pulse rate, and oxygen saturation by pulse oximeter. She appeared stable, with a heart rate of 85 bpm, blood pressure 134/80 mmHg, and respiratory rate 14 bpm, but oxygen saturation by pulse oximeter was 85% on both fingers but normal on the anesthesiologist's finger. Oxygen 6 L/min was administered by mask, and no abnormal breathing sounds were detected. In arterial blood gas analysis, oxygen partial pressure was 307 mmHg and oxygen saturation 100% (Table 1). However, the conjunctiva were pale; her lips and fingernails were cyanosed; and the oxygen saturation by pulse oximeter did not rise above 90%. The patient's guardian indicated that she had a 10-year history of grayish lips and fingernails. Although we considered delaying the surgery, the guardian wanted the surgery to proceed and the patient had the advanced gastric cancer accompanied by hemorrhage and perforation.
Before the anesthesia, a 20 G catheter was placed in the radial artery for continuous hemodynamic monitoring and arterial blood gas analysis. After preoxygenation with oxygen at 6 L/min, lidocaine 20 mg, propofol 120 mg, and vecuronium 10 mg were administered, and manual ventilation was performed with oxygen at 6 L/min. However, the patient experienced severe cyanosis, and the oxygen saturation by pulse oximeter decreased to 79%. After the tracheal intubation and controlled ventilation with oxygen at 3 L/min, the pulse oximeter increased to 83-85%. In arterial blood gas analysis after induction, the sampled blood was dark blackish brown, oxygen partial pressure was 224.7 mmHg, and oxygen saturation was 96.6% (Table 1). The difference between oxygen saturation by arterial blood gas analysis and oxygen saturation by pulse oximeter was presumably due to dyshemoglobin. For confirmation, arterial blood gas analysis and hemoglobin analysis using a CO-oximeter (ABL520, Radiometer, Cofenhagen, Denmark) was ordered for the department of laboratory medicine. Arterial blood gas analysis showed normal findings and hemoglobin analysis showed methemoglobin at 18.3% and oxyhemoglobin at 81.5%. Methemoglobinemia had caused cyanosis and low oxygen saturation by pulse oximeter. Ascorbic acid 600 mg mixed in fluid was administered, and methylene blue 1 mg/kg was intravenously injected slowly over 3 minutes. The methemoglobin level reduced to 11.2% after the administration of ascorbic acid and reduced to 4.3% and then 2.3% after methylene administration. Anesthesia was sustained with oxygen at 3 L/min and enflurane 1.5-2.0 vol%. The surgery lasted 5 hours. The intraoperative oxygen saturation by pulse oximeter was 86-93%. Blood pressure and pulse rate were stable. Two U of packed red blood cells were transfused. After the surgery, the patient had a normal recovery of consciousness and spontaneous respiration, but was transferred to the intensive care unit (ICU). In the ICU, oxygen 6 L/min was administered by a Venturi mask. The oxygen saturation by pulse oximeter was 94%. One day after the surgery, we found that the patient had taken daspone 100 mg/day for 30 years for Hansen's disease without her family ever knowing. After the surgery, methemoglobin in the blood went from 8.5% to 11.0%, but was untreated. From postoperative day 3, it was treated under the diagnosis of adrenal apoplexy due to hypotension. She was discharged without complications on postoperative day 30.
Go to : 
Discussion
Methemoglobin forms when hemoglobin's Fe ion oxides from ferrous (Fe2+) to ferric (Fe3+). In healthy adults, a small amount of hemoglobin oxides to methemoglobin and then it is rapidly converted back to hemoglobin such that methemoglobin levels remain below 1%. This balance is maintained by the intracellular enzyme systems, NADH cytochrome-b5 methemoglobin reductase and NADPH methemoglobin reductase [1]. Methemoglobin cannot reversibly bind with oxygen or carbon monoxide because of its additional cation, so it reduces the oxygen-carrying capacity in arterial blood. It also causes a leftward shift of the oxygen dissociation curve, increasing the affinity of the hemoglobin for oxygen and reducing tissue oxygen supply.
Symptoms of methemoglobinemia are primarily related to the level of methemoglobin but are also influenced by other factors, such as the total hemoglobin level and the cardiovascular status and the respiratory function. Usually, at methemoglobin levels below 15%, patients have no clinical symptoms if adequate hemoglobin levels and preserved cardiac output is sufficient. However, when methemoglobin levels are 15-20%, blackbrown blood and cyanosis occur. Higher levels (20-45%) cause headaches, somnolence, tachycardia, lethargy, and dizziness. When it is 45% or above, respiratory difficulty, acidemia, arrhythmia, heart failure, and seizures occur. When above 70%, there is a high risk of death [2].
Methemoglobinemia can be caused congenitally, by certain foods, or by other unknown causes, but usually occurs when the patient is exposed to drugs or chemical substances that disturb the conversion of methemoglobin to hemoglobin and quicken the development of methemoglobin. When discovered in the perioperative period, it is usually due to the exposure of drugs or chemical substances such as: amyl nitrite, ethyl nitrite, sodium nitrite, silver nitrate, bismuth subnitrate, nitroglycerin, quinones, sulfonamide, sulfapyridine, sulfathiazole, aniline dye, acetanilid, aminobenzenes, aminophenol, benzocaine, prilocaine, and phenacetin [3]. The drugs used in anesthetic practice that cause methemoglobinemia are prilocaine, benzocaine, amyl nitrite, nitroglycerine, phenacetin, and sulfonamide. Drug exposure and subsequent perioperative hypoxia may therefore indicate methemoglobinemia.
Dapsone is a sulfone antibiotic used for the treatment of Hansen's disease and other dermatologic diseases, as well as in immunosuppressed patients to prevent pneumocystitis carinii pneumonia or organ transplant patients, especially those intolerant to sulfonamide. Methemoglobinemia is a known side-effect of dapsone [4-6] and the patients of most cases had a history of dapsone-intake. The patient here had a 30-year history of dapsone-intake, although this information was not obtained initially. Dapsone is almost completely absorbed in the gastrointestinal track and passes the enterohepatic circulation. Only 20% of the dosage-intake is excreted into the urine. The plasma half-life varies from 10 to 80 hours and is dose-dependent.
The pulse oximeter uses 2 wavelengths, 660 nm and 990 nm, to calculate oxygen saturation because the absorbance difference between oxyhemoglobin and deoxyhemoglobin is maximized. Methemoglobin has the same absorbability as oxyhemoglobin and deoxygemoglobin so can affect oxygen saturation measurements by pulse oximeter [7]. Low methemoglobin levels slight decrease oxygen saturation measurements to a limit of 85%, even with fatal methemoglobin levels. Therefore, the pulse oximeter cannot monitor methemoglobin levels. Arterial blood gas analysis calculates oxygen saturation based on arterial oxygen tension and the temperature and may not reflect actual oxygen saturation. Methemoglobinemia diagnosis includes cyanosis; low arterial oxygen saturation that cannot be explained by a problem in the respiratory system or cardiovascular system; and when the normal oxygen partial pressure in the arterial blood gas analysis does not align with the oxygen saturation by the pulse oximeter. Oxygen carrying status is most accurately measured by analysis of all hemoglobin types with a CO-oximeter [7]. Methemoglobinemia may cause blackish brown blood to remain so after exposure to air. Congenital, chronic methemoglobinemia involves a family history or exposure to drugs and toxins, which could be captured in an accurate history. Here, before anesthesia the patient showed cyanosis and low oxygen saturation on the pulse oximeter, which did not align with the arterial blood gas analysis. However, the diagnosis of dyshemoglobins was delayed because of the patient refusal to share her medical history.
Methemoglobin levels were 18.3% initially, and the patient had asymptomatic cyanosis and was hemodynamically stable, but she was given immediate treatment. Despite adequate manual ventilation with oxygen at 6 L/min during anesthetic induction, the patient developed worsened cyanosis and showed decreased oxygen saturation by pulse oximeter to 79%. The eldely patient had diabetes, hypertension, and anemia, potentially indicating ischemic heart disease or impairment in the oxygen-carrying capacity. Ascorbic acid and methylene blue treatment reduced methemoglobin levels to 11.2%, 4.3%, followed by maintenance of hemoglobin level with 2 U of packed red cells.
Cyanosis and 85% oxygen saturation on pulse oximeter before anesthesia could indicate high methemoglobin levels. Despite adequate preoxygenation and manual ventilation with oxygen at 6 L/min anesthesia reduced oxygen saturation by pulse oximeter to 79% and the cyanosis worsened. The anesthetics propofol, vecuronium, and enflurane are not oxidants that develop methemoglobin, although local anesthetics like prilocaine and benzocaine do, and it is uncertain that lidocaine is a oxidant [8]. After tracheal intubation and manual ventilation with oxygen at 6 L/min, the pulse oximeter went up to 84-85% and cyanosis decreased. Therefore, reduced oxygen saturation during anesthetic induction resulted from normal respiratory depression and not anesthetic drugs [8]. In healthy patients, the slight respiratory depression and reduced arterial oxygen partial pressure caused respiratory depressants may be ignored, but in methemoglobinemia, they may cause seriously low oxygen saturation.
Methemoglobinemia treatment is immediate intravenous administration of methylene blue (1-2 mg/kg) and the removal of toxins or drugs that could cause it. Methylene blue acts as a cofactor of methemoglobin reductase and accelerates the enzymatic breakdown of methemoglobin. Methylene blue may be readministered every 30-60 minutes [9]. Higher levels (4-15 mg/kg/day) can directly oxidize hemoglobin to methemoglobin and cause hemolytic anemia in glucose-6-phosphate dehydrogenase deficient patients. Methemoglobin levels above 30% should be treated, but methemoglobin levels below 15% may seem clinically insignificant. However even when the methemoglobin level is low, treatment is recommended for patients with symptoms or underlying diseases with decreased oxygen-carrying capacity such cardiac disease, pulmonary disease, carbon monoxide intoxication, anemia, or acidosis [10]. Life-threatening methemoglobinemia unresponsive to methylene blue may occasionally respond to ascorbic acid as well, although ascorbic acid works slowly. Other reported treatments are homogeneous transfusion, exchange transfusion, and hyperbaric oxygen treatment.
In conclusion, methemoglobinemia is rare, but can be caused by drugs or chemical substances. The anesthesiologist should perform pulse oximeter and arterial blood gas analysis on patients with cyanosis. When the two results do not align, or when the oxygen saturation by pulse oximeter does not align with the clinical manifestations, methemoglobinemia should be suspected. The presence of dyshemoglobin should be confirmed by performing a hemoglobin analysis with a CO-oximeter. Methemoglobinemia should be treated with methylene blue when necessary according to methemoglobin level, symptom, and underlying diseases.
Go to : 




 PDF
PDF Citation
Citation Print
Print


 XML Download
XML Download