Abstract
Venous air embolism (VAE) during intestinal endoscopy is a rare complication. We report a case of cardiovascular collapse due to VAE confirmed by transesophageal echocardiography (TEE) during intraoperative intestinal endoscopy. TEE detected air bubbles in the left ventricle up to 1 hour after the event. When a patient deteriorates during endoscopic procedures, VAE and possible paradoxical air embolism (PAE) should be suspected. This case demonstrates that TEE can play an important role in diagnosing and managing an air embolism in anesthetized patients. In addition, this case demonstrates that PAEs may occur longer than expected after recovery from VAE-induced cardiovascular collapse.
Clinically significant venous air embolism (VAE) during intestinal endoscopy is rare compared to the occurrence of VAE in other clinical settings, such as neurosurgery in the sitting position or liver surgery [1,2]. For the occurrence of an air embolism during endoscopy, intestinal air has to enter the systemic venous system. After occurrence of a VAE, a paradoxical embolism can occur if the entrapped air is delivered to the left ventricle through the pulmonary circulatory system or intracardiac shunting.
The amount and the rate of air entry and patient factors such as presence of collateral vessels or a shunt can affect the clinical outcome of VAE.
The Kasai operation (hepatoportojejunostomy) is one of the risk factors for VAE during endoscopy [2]. Furthermore, hepatic disease can cause intrapulmonary shunting, which is a potential route for venous air to the left ventricle.
There have only been 2 cases reported of fatal VAE during endoscopy in patients who had undergone a Kasai operation [3,4]. We present a case of cardiovascular collapse due to VAE, and subsequent prolonged paradoxical air embolism (PAE) during intraoperative endoscopy in a 17-year-old female who had undergone a Kasai procedure. VAE and PAE were confirmed by transesophageal echocardiography (TEE).
A 17-year-old female patient (height 161 cm, weight 45 kg) who had undergone the Kasai procedure for biliary atresia, was scheduled for an exploratory laparotomy and intraoperative endoscopy to find a focus of gastrointestinal bleeding. Three months earlier, she underwent exploratory laparotomy because of hematochezia, but there was no active bleeding focus identified. Therefore, the only surgery performed was a wedge resection of an area of blue-colored edematous small bowel wall.
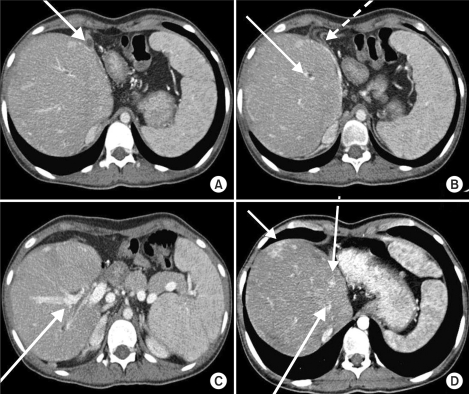
Her preoperative laboratory studies were unremarkable except for a hemoglobin of 8.8 g/dl. Workup for von Willebrand disease was negative. On preoperative bleeding scan (Tc-99m-RBC), there was a suspected bleeding focus at the proximal jejunum, but on 6-hour-delayed imaging, there was no focal bleeding site seen. Preoperative abdominal computed tomography (CT) revealed distorted liver architecture with diffuse vascular sweeping and massive fibrosis (Fig. 1).
Anesthesia was induced without premedication, using intravenous thiopental (225 mg). After neuromuscular blockade was achieved with rocuronium, the trachea was intubated using a 6.0 mm cuffed endotracheal tube. Anesthesia was maintained with 2.0-3.0 vol% sevoflurane. Ventilation was mechanically performed without positive end-expiratory pressure (PEEP) at a tidal volume of 430 ml and a rate of 11 breaths/min. The end-tidal carbon dioxide concentration (ETCO2) was maintained between 30-32 mmHg. Venous access was established using the right external jugular vein and an 18 gauge needle.
Monitoring consisted of noninvasive blood pressure, electrocardiography (ECG) (lead II), pulse oximetry (SpO2), and ETCO2.
Following 30 minutes of adhesiolysis, the endoscope was inserted into the jejunum. The Levin tube was clamped and the bowel was insufflated with pressurized air for a better endoscopic view. Air insufflation pressure was not monitored. Endoscopic examination identified an ulcer and oozing at the site of the proximal hepatojejunostomy.
Immediately after discovery of the ulcerative lesion, the ETCO2 abruptly decreased from 30 mmHg to 17 mmHg and severe bradycardia occurred within a few seconds. The QRS complex widened and ST elevation was observed. After the surgeon was notified, manual bagging with 100% oxygen was begun and atropine (0.5 mg) was injected. The insufflated bowel was decompressed, and the patient was changed from the supine position to the Trendelenburg position on suspicion of air embolism. Her blood pressure could not be measured and carotid arterial pulse was not detected.
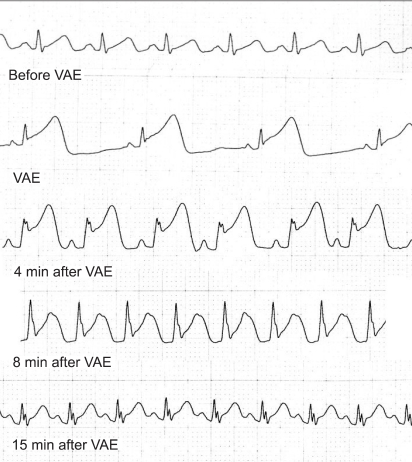
Resuscitation was initiated with external cardiac compression, epinephrine injection, and fluid administration. Thirty seconds later, the sinus rhythm reappeared, but the ST segment remained elevated for 10 minutes after cardiovascular collapse (Fig. 2). Invasive arterial blood pressure monitoring by cannulation of the right radial artery revealed a pressure of 90/58 mmHg. Arterial blood gas analysis at the point of arterial cannulation showed the following values: pH 7.28, PaCO2 45 mmHg, PaO2 402 mmHg with FiO2 of 1.0. Dopamine (5 mcg/kg/min) and nitroglycerin 0.5 (mcg/kg/min) were infused.
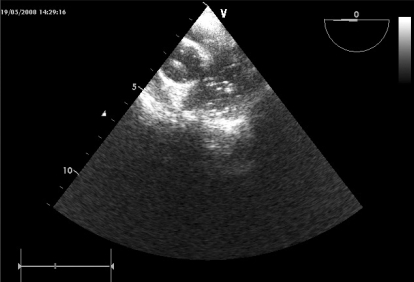
About 30 minutes after the event, a transesophageal echocardiography (TEE) probe was inserted. Air bubbles were observed in both ventricles, the aorta, and the pulmonary artery, without regional cardiac wall motion abnormality (Fig. 3).
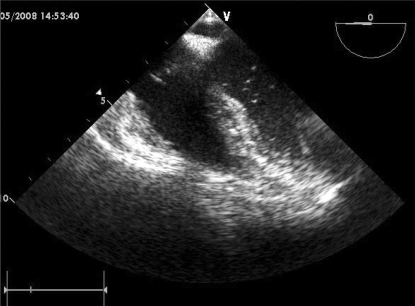
No right-to-left intracardiac shunt was found by color Doppler echocardiography. Although the number of air bubbles decreased gradually, bubbles were continually observed for 1 hour in the pulmonary vein, left atrium, and ventricles (Fig. 4).
To decrease possible systemic air embolism complications, local head cooling and the Trendelenburg position were maintained. Blood pressure was also maintained within upper normal ranges. Arterial blood gas values determined 75 minutes after the event were as follows: pH 7.31, PaCO2 39 mmHg, PaO2 174 mmHg with FiO2 of 0.5.
After the patient's vital signs were stabilized, the endoscopic examination was briefly continued, but no active bleeding focus was found. During the remaining operative period, no more air bubbles were seen by TEE and her vital signs remained stable. A suspicious area of blue-colored bluish small bowel wall was resected.
The patient was transferred to the intensive care unit after surgery. The trachea was extubated about 1 hour after arrival, after confirmation of adequate breathing and stable hemodynamics. There were no neurologic symptoms or signs. There were no abnormalities on MRI and EEG. The resected bowel showed focal submucosal hemorrhages and angiodysplasia. The patient was discharged without significant complications on the eleventh postoperative day.
VAE, now commonly reported with many surgical procedures, has rarely been reported in association with gastrointestinal endoscopy [1]. A survey of members of the American Society for Gastrointestinal Endoscopy in 1976, which encompassed 211, 410 upper endoscopies, did not find any episode of air embolism. A MEDLINE search of English language publications from 1966 through December 2008 identified only 19 cases [1,2,5].
VAE risk factors during endoscopy include a blind intestinal limb such as the Roux-en-Y limb, and directly-exposed large veins which are usually present in gastrointestinal varices or after the Kasai procedure. There are 2 case reports of VAE in patients who underwent the Kasai operation. Lowdon et al. reported on a 4-year-old girl with fatal VAE during gastrointestinal endoscopy. They postulated that air under pressure dissected through diseased liver tissue into a large hepatic vein lying just below the denuded liver surface [4]. Anesthesia was maintained with 60% N2O in oxygen, which could cause expansion of the embolism. In addition, her autopsy found a patent foramen ovale and air in the coronary artery. The second case involved a 10-year-old girl who had undergone a Kasai procedure and died during percutaneous endoscopic manipulation. The autopsy revealed a massive air embolism and varices of the jejunal loop [3]. Our patient had also undergone the Kasai procedure, and air embolism occurred during endoscopy. However, she survived without any morbidity. We did not use N2O, and immediately managed the cardiovascular collapse because of suspicion of VAE. In addition, the relative amount of entrapped air in our patient might have been smaller than the amount in the other patients, because our patient is larger.
For air to enter a blood vessel, a pressure gradient must be present. The common underlying mechanism of venous air/gas entrainment involves subatmospheric pressure at the site of air entry and/or pressurized air or gas being forced into the venous circulation [6]. For the occurrence of PAE during endoscopy, several requirements must be fulfilled. First, there must be an interruption of the intestinal barrier so that the gas in the visceral lumen can enter the blood vessel. Second, the entrapped air in the portal vein must be transported to the systemic venous system, or the air directly enters the systemic venous system. Third, the air in the venous system passes through or bypasses the pulmonary bed, which can occur by several mechanisms, including intracardiac or intrapulmonary shunting, or incomplete filtration of the air embolus by the pulmonary capillaries.
PAE occurs when the air in the right heart passes through some route to the left ventricle. The most common channel for PAE is a patent foramen ovale that is present in 10-35% of the population [7]. Air may also reach the arterial system through pathological dilatation of intrapulmonary vessels normally present in some people (but more frequently seen in patients with end-stage liver disease) [8]. Hopkins et al. found that 47% of patients with chronic liver disease had intrapulmonary right-to-left shunting, as determined by contrast echocardiography [9]. Another study showed that 18 (9.1%) of 88 biliary atresia patients ranging in age from 8 months to 16 years of age had intrapulmonary shunting, and an intrapulmonary shunt can lead to a life-threatening complication in postoperative biliary atresia patients [10].
In our patient, although the bowel was immediately decompressed after VAE was suspected, air bubbles were continually observed in the left ventricle for 1 hour. The air bubbles retained in the pulmonary vein and the left atrium continually entered the left ventricle. The exact amount of air necessary to cause significant hemodynamic disturbances or death has not been determined; however, a bolus of 100 to 300 ml of air can be lethal in an adult human [5,11]. In the canine model, the lung cannot filter microbubbles from the venous system when the rate is greater than 0.3 ml/kg/min [6].
Although clinical signs like "mill-wheel" murmur or a decrease of ETCO2 are useful for detection of VAEs, TEE is widely regarded as the most sensitive means of detection, and can identify bubbles as small as 20-50 µm in diameter [12,13]. TEE is much more effective than other technology at detecting PAEs, because it is hard to assess the passage of air bubbles to systemic circulation by other methods. In addition to confirmation of air in both ventricles by TEE, we also found that the air in the left heart could be observed for approximately 1 hour after the event. These findings suggest that even after hemodynamic stabilization following VAE, patients should be managed to prevent possible PAE. TEE is very helpful for the management of the patient with VAE, because it provides a diagnosis along with valuable information such as cardiac function, the amount of air in the heart, the presence of an intracardiac shunt, and the occurrence of PAE.
The primary goal in treatment of VAE is the prevention of further air entry and, if possible, a reduction in the volume of air entrained. When VAE is suspected, the surgeon should immediately be notified [14], N2O should be discontinued, and ventilation should be performed with 100% O2. Because it is 34 times more soluble in blood than nitrogen, N2O will rapidly diffuse into trapped air bubbles and dramatically increase the size of the embolus. Also, the operative site should be lowered to below the level of the heart whenever possible. Although the air entrance point, the jejunal site, was above the level of our patient's heart when she was in the Trendelenburg position, we thought that the driving force causing air to enter from the intestine was the insufflated pressure, not subatmospheric pressure. Therefore, if the insuffluation was stopped, the position of the patient would have little effect on air entrapment. The Trendelenburg position could also be helpful in stabilizing the hemodynamics by increasing venous return. Additionally, the circulation of a large volume of air could be delayed by placing the patient in the Trendelenburg position. Adornato et al. also has suggested that the Trendelenburg position can improve hemodynamics [15]. Finally, pharmacologic support, including inotropic drugs such as epinephrine to improve cardiac output, can be helpful [14].
PAE can have catastrophic sequelae. One of the most serious complications is coronary embolization. It induces electrocardiographic changes typical of ischemia and infarction, dysrhythmias, and myocardial suppression. To minimize complications, pharmacologic support, such as nitroglycerin, dopamine, and norepinephrine can be helpful. Other possible complications are neurologic sequelae, which lead to various symptoms from mild headache to hemiparesis or coma. To reduce the chance and rate of air embolus entering the cerebral circulation, we changed the position of the patient to the Trendelenburg position. Ischemic damage to the brain can be minimized by maintaining the cerebral perfusion pressure. Increasing the systemic blood pressure along with reducing the cerebral metabolic rate by cooling the head may be helpful.
In our patient, the intestine was insufflated with medical air as in a conventional gastrointestinal endoscopy and not CO2. Furthermore, the insufflated pressure was not monitored.
In our opinion, pressure-regulated CO2 would be a better choice for insufflation in this patient, because plasma CO2 levels can be controlled with mechanical ventilation, and this patient had risk factors for VAE such as liver-disease-associated collateral vessels, previous Kasai operation, and intestinal bleeding.
In summary, VAE can occur during an endoscopic procedure, especially in patients with risk factors, and PAE can occur even after recovery of air-embolism-induced cardiovascular collapse. TEE has an important role in diagnosing and managing VAE and PAE in anesthetized patients.
References
1. Holzman RS, Yoo L, Fox VL, Fishman SJ. Air embolism during intraoperative endoscopic localization and surgical resection for blue rubber bleb nevus syndrome. Anesthesiology. 2005; 102:1279–1280. PMID: 15915042.

2. Green BT, Tendler DA. Cerebral air embolism during upper endoscopy: case report and review. Gastrointest Endosc. 2005; 61:620–623. PMID: 15812425.

3. Desmond PV, MacMahon RA. Fatal air embolism following endoscopy of a hepatic portoenterostomy. Endoscopy. 1990; 22:236. PMID: 2242745.

4. Lowdon JD, Tidmore TL Jr. Fatal air embolism after gastrointestinal endoscopy. Anesthesiology. 1988; 69:622–623. PMID: 3177925.

5. Mittnacht AJ, Sampson I, Bauer J, Reich DL. Air embolism during sigmoidoscopy confirmed by transesophageal echocardiography. J Cardiothorac Vasc Anesth. 2006; 20:387–389. PMID: 16750742.

6. Herron DM, Vernon JK, Gryska PV, Reines HD. Venous gas embolism during endoscopy. Surg Endosc. 1999; 13:276–279. PMID: 10064764.

7. Schwarz G, Fuchs G, Weihs W, Tritthart H, Schalk HV, Kaltenbock F. Sitting position for neurosurgery: experience with preoperative contrast echocardiography in 301 patients. J Neurosurg Anesthesiol. 1994; 6:83–88. PMID: 8012178.

8. Krowka MJ, Cortese DA. Hepatopulmonary syndrome. Current concepts in diagnostic and therapeutic considerations. Chest. 1994; 105:1528–1537. PMID: 8181347.
9. Hopkins WE, Waggoner AD, Barzilai B. Frequency and significance of intrapulmonary right-to-left shunting in end-stage hepatic disease. Am J Cardiol. 1992; 70:516–519. PMID: 1642191.

10. Sasaki T, Hasegawa T, Kimura T, Okada A, Mushiake S, Matsushita T. Development of intrapulmonary arteriovenous shunting in postoperative biliary atresia: evaluation by contrast-enhanced echocardiography. J Pediatr Surg. 2000; 35:1647–1650. PMID: 11083444.

11. Toung TJ, Rossberg MI, Hutchins GM. Volume of air in a lethal venous air embolism. Anesthesiology. 2001; 94:360–361. PMID: 11176104.

12. Derouin M, Couture P, Boudreault D, Girard D, Gravel D. Detection of gas embolism by transesophageal echocardiography during laparoscopic cholecystectomy. Anesth Analg. 1996; 82:119–124. PMID: 8712385.

13. Dion YM, Levesque C, Doillon CJ. Experimental carbon dioxide pulmonary embolization after vena cava laceration under pneumoperitoneum. Surg Endosc. 1995; 9:1065–1069. PMID: 8553204.

14. Palmon SC, Moore LE, Lundberg J, Toung T. Venous air embolism: a review. J Clin Anesth. 1997; 9:251–257. PMID: 9172037.

15. Adornato DC, Gildenberg PL, Ferrario CM, Smart J, Frost EA. Pathophysiology of intravenous air embolism in dogs. Anesthesiology. 1978; 49:120–127. PMID: 686416.

Fig. 1
Preoperative CT image of the liver. (A) Left lobe atrophy, (B) Portal thrombosis, right portal vein sweeping (dotted line), (C) Hepaticojejunostomy site, (D) Multifocal arteriovenous shunt.




 PDF
PDF Citation
Citation Print
Print


 XML Download
XML Download