Abstract
Ehlers-Danlos syndrome (EDS) is a rare inherited disorder of the connective tissue that is characterized by hyperextensible skin, hypermobile joints and abnormalities of the cardiovascular system. A 15-year-old girl with Ehlers-Danlos syndrome underwent thoracolumbar surgery for deformity correction. After surgery, an abdominal aortic rupture occurred, and she complained of abdominal distension had an abdominal circumference of 80 cm. Abdominal computed tomography revealed a pseudoaneurysm and a large hematoma at the retroperitoneum. She died of a massive hemorrhage during subsequent abdominal aortic surgery.
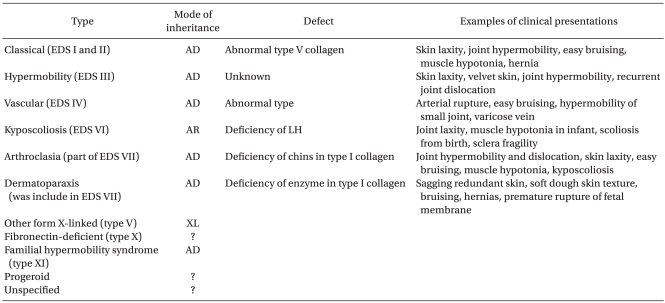
Ehlers-Danlos syndrome (EDS) is a group of very rare inherited connective tissue disorders that are categorized into six major types: classical, hypermobility, vascular, kyposcoliosis, arthroclasia, and dermatoparaxis. A deficiency of lysyl hydroxylase is characteristic of the kyposcoliosis type, whose main clinical features involve hypotension, moderate joint laxity, skin hyperelasticity, extreme hyposcoliosis, and blood vessel fragility, etc. The vascular fragility in patients with EDS syndrome undergoing scoliosis surgery can result in aortic damage or cause platelet defects associated with coagulation disorders in some patients with the vascular type, which poses a potential danger of massive hemorrhage. We report a death caused by a rupture of the abdominal aorta after scoliosis surgery at our hospital, with review of the relevant literature.
A 15-year-old, 42 kg, 151 cm, female patient presented with congenital scoliosis (Thoracic 4-Lumbar 5) for scoliosis correction surgery. On the preoperative examination, her forced expiratory volume in 1 second (FEV1) was 1.80 L (67%), and her forced vital capacity (FVC) was 1.96 L (69%), indicating a restrictive ventilatory defect. Three years ago, she had been diagnosed with Ehlers-Danlos syndrome (EDS). On preoperative echocardiography, no cardiovascular disorders, such as mitral valve prolapse or aortic dilation, were detected, so the type of EDS was presumed to be the kyposcoliosis type. The blood test before surgery revealed the following: hemoglobin (HB) 10.9 g/dl, hematocrit (HCT) 34.8%, and platelets (PLT) 219,000/ul. The coagulation disorder test showed a prothormbin time (PT), prothrombin time-international normalized ratio (PT-INR) and activated partial thromboplastin time (aPTT) of 11.8 s, 33.1 s and 33.1 s, respectively. On the preoperative airway evaluation, the interincisional distance at the maximal mouth opening was 4 cm, the thyro-mental distance was 5 cm, but the Mallampati classification was Class II and the neck mobility was normal. There was neither gingival bleeding nor periodontal disease.
Without premedication, the patient arrived conscious in the operating room (OR). Her arterial pressure and heart rate was 120/80 mmHg and 102/min, respectively. The motor evoked potential test was scheduled to be conducted in order to prevent intra-operative neurological damage, so total intravenous anesthesia was performed to avoid a decrease in the amplitude of the evoked potential (EP) or an increase in the refractory period due to the anesthetics. Through face mask ventilation, de-nitrification was performed with 100% O2. Anesthesia was achieved with 30 mg of 2% lidocaine injected intravenously and 2% propofol (Fresofol®, Fresenius Kabi, France) injected with a target effect-site concentration of 4 µg/ml using a target controlled infusion machine (Orchestra®, Fresenius Vial, France). After confirming the loss of the palperbral/eyelid reflex, remifentanil (Ultiva®, GlaxoSmithKline, UK) was administered with a target effect-site concentration of 4.5 ng/ml. The Marsh and Minto model were used for a pharmacokinetic model of propofol and remifentanil.
Endotracheal intubation was performed after injecting rocuronium 0.6 mg/kg intravenously. Patients with EDS are quite vulnerable to airway damage so the intracuff pressure was maintained between 25-35 cmH2O. Remifentanil was maintained at a target effect-site concentration of 4.5 ng/ml for stable blood pressure (BP) during intubation in order to prevent an increase in BP from damaging the fragile blood vessel wall. After the induction of anesthesia, a 22-G catheter was placed into the radial artery to measure BP continuously, while a 7 Fr double lumen catheter was placed to the right internal jugular vein to measure the central venous pressure, and as an intravenous route for a massive transfusion. To maintain the appropriate anesthesia depth, the effect-site concentration of remifentanil was set to 3.0-4.5 ng/ml, while the effect-site concentration of propofol was set to 3.0-4.5 µg/ml in the target controlled infusion machine. To monitor the sedation depth, a bispectral index monitor (A-2000®, Aspect Medical Inc., USA) was attached to the patient and maintained within the range of 40-60. Her tidal volume (TV) and tidal rate (TR) was 330 ml and was 14/min, respectively. After the onset of anesthesia, she had a severe hemorrhage. Despite continuous transfusion, maintenance of the appropriate BP failed, so dopamine at a dose of 10 µg/kg/min and norephinephrine at a dose of 0.05 µg/kg/min were administered continuously until the systolic pressure of BP was maintained at 80-90/50-55 mmHg. The total operation time was 7 hours 50 minutes, and the anesthetic time was 9 hours 50 minutes. The patient was transferred to the intensive care unit, with the endotracheal tube maintained. The estimated/presumptive amount of blood loss was 8,000 ml. The total amount of transfusion consisted of 7,500 ml of packed red blood cell, 11 units of fresh frozen plasma (FFP), 500 ml of crystalloid solution, and 16,950 ml of a colloid solution. The urine output was 900 ml.
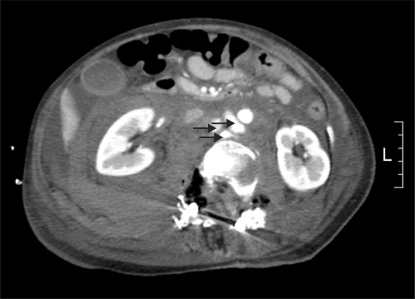
After surgery when the patient reached consciousness, she complained of pain at the surgical site and a feeling of abdominal distension. She was infused with dopamine at 5 µg/kg/min and norephinephrine at 0.05 µg/kg/min with the BP maintained at 90/40 mmHg. The blood test results revealed the following: hemoglobin level of 12.7 g/dl, hematocrit of 38.9% and platelet count of 51,000/ul. Arterial blood gas analysis (ABGA) showed a pH of 7.26, pCO2 of 39 mmHg, PO2 of 196 mmHg, HCO3- of 17.7 and mEq/L at FiO2 of 0.5. The prothrombin time-international normalized ratio (PT-INR) was 0.39 and the partial thromboplastin time (PTT) was 73.6 sec. A sudden, abnormal increase in her abdominal circumference after surgery was easily recognizable and her waist measurement was 80 cm. Through the drainage tube placed during surgery, 500 ml and 300 ml of drainage was performed on postoperative day 1 and 2, respectively. On postoperative day 2, the drainage tube was withdrawn, but the abdominal pain and feeling of abdominal distension worsened on day 3 after surgery. The blood test results revealed the following: hemoglobin level of 10.4 g/dl, hematocrit of 31.0% and platelet count of 79,000/ul. Arterial blood gas analysis (ABGA) showed a pH of 7.37, pCO2 of 40 mmHg, PO2 of 103 mmHg, HCO3- of 22.8 and mEq/L at FiO2 of 0.4. The patient's abdominal circumference was 80 cm, showing no change. Abdomen computed tomography (CT) performed to determine the cause of the abdominal distension detected a pseudoaneurysm, 2.7 × 1.1 cm, 1.4 × 0.8 cm, 1.0 × 0.6 cm (maximum length 4 cm) in size, in the right renal hilum layer, and a large hematoma in the retroperitoneal cavity. A decision was made to carry out emergency surgery to remove the hematoma and to restore the abdominal aorta. Anesthesia was induced in the same manner as before. For the maintenance of anesthesia, the effect-site concentration of remifentanil was maintained in the target controlled infusion machine at 2.0-3.0 ng/ml while that of propofol was at 2.0-3.5 µg/ml. At a FiO2 of 1.0, her TV and TR was 350 ml and 12/min, respectively.
ABGA, which was performed at a FiO2 of 1.0 immediately after the beginning of surgery, showed a pH of 7.41, pCO2 of 34.6 mmHg, PO2 of 346 mmHg and HCO3- of 21.5 mEq/L. After the beginning of surgery, a severe hemorrhage developed and a transfusion was started. Fifty minutes after the beginning of surgery, her BP decreased to 80/50 mmHg, so epinephrine was administered twice with 30 µg at each time but BP maintenance failed. Epinephrine was then infused continuously at 0.02 µg/kg/min and with an increase to 0.05 µg/kg/min after 5 minutes. The ABGA results, performed at a FiO2 1.0, two hours after the beginning of surgery, showed a pH of 7.23, pCO2 of 29 mmHg, PO2 of 450 mmHg, HCO3- of 12.4 mEq/L, and Ca2+ 0.41 mmol/L. Therefore, calcium gluconate was injected intravenously with an initial dose of 1 g and afterwards, 1 g twice and 2 g 5 times were also administered.
Three hours after the beginning of surgery, cardiac arrest developed, so epinephrine was administered with 200 µg and 300 µg. The patient showed no reaction, epinephrine 1 mg, calcium gluconate 1 g, and sodium bicarbonate 40 mEq were administered twice, but the cardiac arrest persisted. Pentotal sodium 200 mg and atropine 0.5 mg were administered and a cardiopulmonary bypass was performed immediately. An attempt was made to restore the defect region with Boretex, but the blood vessel kept tearing every time a suture was made. This made suturing impossible and resulted in the decision to end the cardiopulmonary bypass. The surgeon explained the patient's condition to her parents and terminated the operation at 8 hours and 55 minutes after the beginning of surgery. The patient was transported to the intensive care unit with the endotracheal tube maintained, and pronounced dead. The presumptive amount of blood loss was 18,000 ml. The total amount of transfusion consisted of 12,000 ml of packed red blood cells, 3 units of fresh frozen plasma (FFP), 10 units of platelet concentrates, 500 ml of crystalloid solution, and 17,400 ml of colloid solution. The urine output was 380 ml.
Ehlers-Danlos Syndrome (EDS) is a rare inherited disorder of the connective-tissue that was first noted by Hippocrates as bruises and bleeding around 400 BC. Its clinical features were described by Edvard Ehlers In 1901, and by Hrei-Alexander Danlos in 1908 [1]. The prevalence is approximately 1 case in 5,000 people. The symptoms involve hypermobility of the joints, fragility and hyperelasticity of the skin, poor wound healing (easy bruising and scaring), musculoskeletal problems, vulnerability to osteoarthritis, etc. A defect in type-III collagen can also cause spontaneous rupture of the gastrointestinal tract, uterus, blood vessel, etc. [2]. This hereditary disorder is caused by a mutation of an amino acid in the gene of the fibrous protein COL3A1, which leads to a deficiency in type-III collagen synthesis [3]. Since the 1960s, the classification of EDS attempted to recognize its diverse types, and in 1998, Beighton and other researchers proposed a simpler classification, as shown in Table 1 [4]. In particular, the kyposcoliosis type is characterized by a deficiency of an enzyme called lysyl hydroxylase and its main clinical features involve muscle hypotonia, skin hyperextensibility, moderate joint laxity, severe kyposcoliosis and blood vessel fragility [5]. On the other hand, the vascular type is caused by a structural defect in type-III collagen due to mutations in the COL3A1 gene, which carries a risk of arterial rupture, gastrointestinal perforation and uterine rupture [3]. The vascular type is inherited in an autosomal dominant manner, and the primary diagnosis of this type is characterized by thin, translucent skin, and arterial, intestinal, and/or uterine fragility, as well as easy bruising and characteristic facial appearance, etc. The secondary diagnostic manifestations are acrogeria, hypermobility of the small joints, tendon or muscle rupture, clubfoot, early onset of varicose veins, aneurysms, carotid-cavernous sinus fistula, pneumothorax or hemorthorax, gingival crater, family history of sudden death, etc. [4]. The present case showed the typical characteristics of the kyposcoliosis type, such as systemic joint laxity, severe muscle hypotonia since birth, and progressing kyposcoliosis, but not sclera fragility. Moreover, there were no clinical manifestations of the vascular type including thin, translucent skin, and characteristic facial appearance, acrogeria, early onset of varicose veins, gingival crater, etc. The diagnosis of EDS was made initially based on her family history and clinical symptoms, which alone is difficult to judge. Therefore, in order to make a definite diagnosis, either biochemical testing to identify the mutation of the collagen fiber molecule on cultured dermal fibroblasts, or sequence analysis using DNA should be performed [3]. However, we did not apply either test to the patient. Therefore, it was difficult to secure a definite diagnosis.
In our patient, the kyposcoliosis type was assumed before surgery. However, the blood vessels were so fragile that their restoration failed during anesthesia or after aortic surgery in the second operation. Therefore the possibility of the vascular type should not be excluded. The most critical problems in general anesthesia arise from either endotracheal intubation vulnerability or vascular fragility. Patients with EDS may have difficulty in endotracheal intubation due to cervical vertebra problems, gingival bleeding, or oral pharyngeal tissue fragility. In addition, the increase in BP upon intubation may damage the fragile vessel wall. Therefore, it is necessary to first check for an abnormality of the vertebra, dental history of gingival bleeding or periodontal disease, and difficulty in endotracheal intubation. The cardiac function should be examined to confirm the association with cardiovascular system abnormalities, such as mitral valve prolapse, aneurysm, etc [6]. The possibility of a massive hemorrhage during anesthesia of patients with EDS should be considered. An intramuscular injection should be avoided and the bleeding tendency also should be noted when manipulating a surgical apparatus in the nose or esophagus. Upon direct laryngoscopy, the possibility of trauma must be minimized while the prospect of hematoma should be considered when placing a catheter in the artery or vein. In addition, a low airway pressure must be maintained during mechanical respiration due to the high risk of pneumothorax. Local anesthesia is not recommended for patients with EDS because an abnormality of the musculoskeletal system makes surgery difficult, there is vascular fragility and a risk of hematoma due to coagulation disorders [7]. In our case, after successive failures to placing a central venous catheter (CVC) because of the vascular flexibility, the catheterization was achieved only after by ultrasonography. The airway pressure was maintained at <15 cmH2O to reduce the risk of pneumothorax.
As in the present case, patients with EDS are at high risk of neurological and vascular damage when undergoing back surgery. Vogel and Lubicky [8] reported side effects in 4 patients after undergoing back surgery: restoration of a damaged aorta was required in 2 patients with quadriplegia, 1 patient had asthenia on the leg and ankle, and 1 patient had an isolation of the anterior segmental artery. Akpinar et al. [9] reported their experience of restoring damaged aorta and iliac vein in one of 5 EDS patients undergoing surgery for spinal deformities. There were no special neurological abnormalities in our patient except for the vascular abnormality detected after the first operation. However, in our case, there was the possibility of direct surgical injury on a preoperatively existent aortic lesion, or the possibility of secondary damage by a spinal/pedicle fixation instrument. Considering the results of the present case, more careful attention should be paid to vascular damage when performing operations on patients with EDS through close collaboration with the attending surgeons, which can help detect early vascular damage and take the appropriate actions. In particular, in patients scheduled to undergo spinal surgery with a high risk of vascular damage, it is advisable to carry out a biochemical or DNA test to make a definite diagnosis of the type of EDS.
References
1. Parapia LA, Jackson C. Ehlers-Danlos syndrome - a historical review. Br J Haematol. 2008; 141:32–35. PMID: 18324963.

2. Germain DP. Clinical and genetic features of vascular Ehlers-Danlos syndrome. Ann Vasc Surg. 2002; 16:391–397. PMID: 12016538.

3. Pope FM, Martin GR, Lichtenstein JR, Gerson B, Rowe DW, McKusick VA. Patients with Ehlers-Danlos type IV lack type III collagen. Proc Natl Acad Sci U S A. 1975; 72:1314–1316. PMID: 1055406.
4. Beighton P, De Paepe A, Steimann B, Tsipouras P, Wenstrup RJ. Ehlers-Danlos syndrome: revised nosology, Villfranche, 1997. Am J Med Genet. 1998; 77:31–37. PMID: 9557891.
5. Sussman M, Lichtenstein JR, Nigra TP, Martin GR, McKusick VA. Hydroxylysine-deficient skin collagen in a patient with a form of the Ehlers-Danlos syndrome. J Bone Joint Surg Am. 1974; 56:1228–1234. PMID: 4373475.

6. Dolan P, Sisko F, Riley E. Anesthetic considerations for Ehlers-Danlos syndrome. Anesthesiology. 1980; 52:266–269. PMID: 7369516.

7. Schwartz JJ. Hines RL, Marschall KE, editors. Skin and Musculoskeletal disease. Stoelting's anesthesia and co-existing disease. 2008. 5th ed. Philadelphia: Churchill livingstone;p. 444.
8. Vogel LC, Lubicky JP. Neurologic and vascular complications of scoliosis surgery in patients with Ehlers-Danlos syndrome. Spine. 1996; 21:2508–2514. PMID: 8923641.

9. Akpinar S, Gogus A, Talu U, Hamzaoglu A, Dikici F. Surgical management of the spinal deformity in Ehlers-Danlos syndrome type VI. Eur Spine J. 2003; 12:135–140. PMID: 12709851.





 PDF
PDF Citation
Citation Print
Print


 XML Download
XML Download