Abstract
Background
The optimal dose infusion of 0.125% bupivacaine via a femoral catheter after total knee replacement (TKR) has not been defined. This study examined various dose infusions of bupivacaine to determine the analgesic quality in patients receiving a continuous femoral nerve block (CFNB).
Methods
Patients were randomized to receive a single-injection femoral nerve block (SFNB) or CFNB performed with 20 ml of 0.125% bupivacaine, followed by a continuous infusion of 0.125% bupivacaine in four groups (n = 20 per group): 1) 0 ml/h (SFNB), 2) 2 ml/h, 3) 4 ml/h, and 4) 6 ml/h. The pain intensity at rest and on knee movement was assessed using a visual analog scale (VAS) for the first 2 postoperative days. The cumulative bolus use of IV patientcontrolled analgesia (PCA) with a morphine-ketorolac combination was evaluated.
Results
A lower cumulative bolus of IV PCA was noted in all CFNB groups compared to SFNB on postoperative days (PODs) 1 and 2, respectively (P < 0.05). Lower VAS scores at rest were observed in the 4 ml/h and 6 ml/h groups than in the SFNB group on PODs 1 and 2, respectively, but only on POD 2 in the 2 ml/h group (P < 0.05). Lower VAS scores on movement were noted in the 4 ml/h than the SFNB group on PODs 1 and 2, but only on POD 1 in 6 ml/h (P < 0.05).
A total knee replacement (TKR) is associated with marked and prolonged postoperative pain. Recently, single-dose and continuous peripheral nerve techniques that block the femoral nerve have been used [1,2]. However, a continuous femoral nerve block (CFNB) leads to the administration of large volumes of bupivacaine with the potential risk of toxicity caused by the accumulation of the drug after prolonged periods of infusion [3]. Therefore, "dose-sparing" infusion techniques are needed. The reported infusion rates of 0.125% bupivacaine that provide effective analgesia vary from 6 [4] to 10 [5] or 12 ml/h [3]. However, lower infusion rates of bupivacaine for FNB in terms of analgesia have not been evaluated, leaving unanswered the question of whether decreasing the infusion rates will still provide adequate analgesia.
CFNB may be beneficial compared to single-injection femoral nerve block (SFNB) because previous studies reported that the duration of the analgesic effect from a SFNB is typically 8 to 24 h [1,6], whereas severe pain after TKR, particularly during physical therapy, may persist through the second day after surgery [7].
CFNB is the standard treatment for postoperative pain after TKR at our institution. To date, an optimal dose infusion of 0.125% bupivacaine via a femoral catheter after TKR has not been defined. This study examined the minimum effective infusion rate of 0.125% bupivacaine (2, 4, and 6 ml/h) for CFNB. The primary aims were the analgesic efficacy, systemic opioid analgesic consumption, and treatment-related side effects among the various doses of femoral infusion used. The secondary aim was to compare the effects of SFNB versus CFNB.
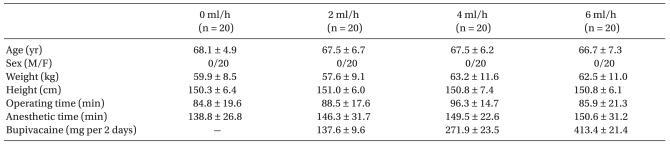
After gaining approval of the Institutional Ethics Committee and written informed consent, 88 ASA physical status I or II female patients scheduled to undergo elective unilateral TKR under spinal anesthesia (SA) were enrolled in this study. Patients who had undergone surgery in the morning were included. There were no male patients during the study period. Patients were excluded if they met any of the following criteria: contraindications to a regional anesthetic technique (e.g., local infection, sepsis, coagulation abnormality), allergy to local anesthetic or morphine, contraindications for NSAID use, preexisting neurological deficit in the lower extremities, and inability to comprehend the pain scales or use IV patientcontrolled analgesia (PCA) device. After surgery in the postanesthesia care unit (PACU), all patients were given an explanation of the visual analog scale (VAS) and were also familiarized with the use of IV PCA. There were no significant differences between the study groups in terms of age, weight, height, anesthetic time and operating time (Table 1). In the anesthetic time, the duration for FNB or femoral catheterization was included.
Patients were randomized to receive SFNB (control group) with 20 ml of 0.25% bupivacaine with epinephrine 1 : 200,000 or CFNB performed with a bolus dose, followed by a continuous infusion of 0.125% bupivacaine in the following four groups (n = 20 per group): 1) 0 ml/h (SFNB), 2) 2 ml/h, 3) 4 ml/h, and 4) 6 ml/h. This prospective study was designed to evaluate the analgesic efficacy and side effect profile of various infusions of 0.125% bupivacaine during CFNB compared to SFNB after TKR. Premedication consisted of 1-2 mg of IV midazolam.
In the patients randomized to SFNB, the femoral blocks were performed before the induction of SA by one investigator experienced in these techniques. With patients in the supine position, the puncture site was located 1-2 cm below the inguinal crease and 1.5-2.0 cm lateral to the femoral artery. The femoral nerve was identified using a nerve stimulator (Innervator®, Fisher&Paykel, New Zealand) via a 50-mm, 22-gauge insulated needle (Stimuplex®; B. Braun, Melsungen, Germany) for precise nerve location. With an initial output of 2 mA, the block needle was advanced cephalad in the sagittal plane at an angle of 30° to the skin until quadriceps femoris muscle contractions were elicited. The position was then judged adequate when an output of ≤0.4 mA at a frequency of 1 Hz still elicited contractions of the quadriceps. After negative aspiration for blood, 20 ml of the local anesthetic solution was injected over a 2-min period. SA was performed immediately after the procedure was accomplished.
In the patients randomized to CFNB, a femoral catheter was placed before inducing SA by one investigator. The femoral nerve was located accurately with an 18 gauge short-beveled cannula (Contiplex® set; B. Braun, Melsungen, Germany) using the same method described for the single-injection technique. Ten ml of normal saline were injected in the sheath before introducing the catheter and a 20-gauge catheter was threaded 10-15 cm in a cephalad direction from the skin. The femoral catheters were tunneled 5 cm subcutaneously and laterally, after which the catheters were secured to the skin with a catheter-locking device (AceLock, Acemedical, Korea), which was then covered with a transparent plastic cover (Ioban™ 2, 3M Health Care, USA). Approximately 2-3 ml of normal saline was injected through the femoral catheter to ensure patency. To verify the correct position of the catheter, 3 ml of contrast media (iopamidol) was injected via the catheter and the location of the catheter tip was confirmed using a C-arm fluoroscope. A printed paper of the pelvis revealed the zone of diffusion of the contrast media and the location of the catheter tip under the fascia. This position was qualified as medial (over the psoas muscle) (Fig. 1A), intermediate (Fig. 1B), or lateral (over the iliacus muscle) (Fig. 1C). The lumbar plexus anatomy and the zones reached by the spread of local anesthetic under the fascia iliaca were reported by Capdevila et al. (Fig. 2) [8].
SA was performed at the L3-4 or L4-5 intervertebral space using a 25-gauge Quinke needle with the patient in the lateral position and the surgical side down. All patients were administered 6-8 mg of hyperbaric bupivacaine 0.5% together with epinephrine 0.05 mg and 0.1 mg of morphine sulfate into the intrathecal space. After obtaining successful SA, a bladder catheter was inserted and surgery was allowed to proceed. The procedure was performed with a tourniquet applied to the thigh and inflated at 300 mmHg. All surgical procedures were performed by the same orthopedic surgeon. No local anesthetics were injected through the femoral catheter during surgery in any of the CFNB groups. Further intraoperative sedation consisted of midazolam 1-2 mg.
At the end of the operation in the CFNB groups, 20 ml of a local anesthetic solution was administered via the catheter. In the PACU, a continuous femoral infusion of 0.125% bupivacaine at a rate of 2, 4, and 6 ml/h, depending on the group allocation, was started one hour after the bolus injection, which was then continued into the postoperative period for 3 or 4 days. The amount of postoperative bupivacaine received by each patient in the first 2 days was noted and the femoral catheters were removed on the 3rd or 4th postoperative day (POD). A sensory block using a pinprick test with a blunt needle on the anterior, medial and lateral aspect in the middle third of the thigh were assessed twice daily by an independent observer. This was confirmed by a lack of or reduced sensation involving the each aspect compared to the same stimulation in the contralateral thigh. The loss of sensation was assessed around the middle third of the thigh in each aspect, but not around the knee because of the presence of extensive dressings around the knee after surgery. A pinprick test in the PACU was performed upon resolution of the spinal anesthetic.
In the PACU, the patients were provided with a IV PCA device (Accumate 1000®, Woo Young Medical, Korea) containing 0.5 mg/ml morphine plus 1.5 mg/ml ketorolac. The PCA system was set at a bolus of 1 ml of the morphine-ketorolac solution on demand, with an 8-min lockout interval and no continuous background infusion. The patient was then asked to press the button whenever she was in pain at a VAS scale of >30 out of 100. The cumulative bolus use of IV PCA (ml) was recorded from the electronic memory of the PCA machine at the measurement times for the first 2 days. The pain intensity at rest and with a standardized motion of the knee (30° of passive flexion) was assessed by the patients using a VAS (0 = no pain and 100 = worst pain) in the PACU, at 6 PM on the day of surgery, and at 9 AM and 6 PM on POD 1 and 2, respectively. The patients received rescue doses of 90 mg of diclofenac IM when dissatisfied with the IV PCA. The rescue doses of diclofenac were recorded at each measuring interval for the first 2 days.
The presence and severity of nausea was assessed using an ordinal scale (0 = no nausea; 1 = mild; 2 = moderate; 3 = severe). Ten mg metoclopramide IV and 8 mg ondansetron IV were prescribed routinely to all patients as antiemetic medication upon arrival to the ward and for the first 2 days. The treatment for pruritus consisted of IM chlorpheniramine 4 mg, and treatment in all cases was initiated upon the patient's request.
All patients were monitored for respiratory depression by an assessment of the respiratory rate. The respiratory rates were assessed by the study anesthesiologist at the measurement times and by the ward nurses every 4 h. A respiratory rate <10 breaths/min was considered to be respiratory depression and was treated systematically with incremental doses of naloxone as required. Supplemental oxygen was not used routinely. However, oxygen 3 L/min was administered via a nasal cannula if at any time their respiratory rate was <10 breaths/min.
The degree of patient sedation was graded on a 4-point scale: 0 = alert; 1 = drowsy; 2 = sleeping, easy to arouse; and 3 = sleeping, difficult to arouse. Significant sedation was defined as a sedation score of 3. Urinary retention could not be assessed because indwelling catheters in the urinary bladder were left in place for approximately 24 hours postoperatively. All data was collected by an anesthesiologist not involved in the administration of anesthesia or in patient care in the PACU.
Statistical analysis was performed using SPSS software (version 12.0, USA). A one-way ANOVA test was performed to compare the patient's age, weight and height, anesthetic time and operating time, and Dunett test was used as a post hoc test. One-way ANOVA and a Dunett test as a post hoc test was used to compare the PCA bolus use and VAS pain scores. A chi-squared test was used to compare the location of the catheter tip, sensory block, side effects and rescue medications. For all statistical analyses, a P value < 0.05 was considered significant.
Of the 88 patients enrolled, 8 were excluded from data analysis for the following reasons: three patients had failed FNB despite successful identification of the femoral nerve with a nerve stimulator; one patient experienced a dislodged femoral catheter on POD 1; one patient frequently used demand doses for pain at a site other than the operated knee because patients undergoing knee replacement surgery frequently have a polyarticular affliction; one patient frequently pressed the demand button due to anxiety and not for pain; one patient showed postoperative delirium on POD 1; and one patient showed inadequate SA. Including the results from these eight patients would have masked the overall effect of the FNB. If a patient was removed from the trial, the same trial was performed on another patient. A final total of 80 patients were distributed equally among the groups.
The location of the catheter tip was verified by a C-arm after catheterization in all CFNB patients. Of 60 patients receiving a femoral catheter, 6 could not obtain printed paper due to a failure of the printer. Therefore, only 54 could have printed paper of the pelvic region. Of these, 3 catheters, one each in 2, 4, and 6 ml/h, showed coiling in the femoral head area, even though the length inserted was 10-15 cm. Fortunately, the FNB occurred in one each in 4 and 6 ml/h. Unfortunately, the block was considered to have failed in one in the 2 ml/h group. Subsequently, the femoral catheter was reinserted with a nerve stimulator in the PACU for the patient, and the success of the femoral block after the bolus injection was confirmed. These 3 patients were excluded from data analysis for the catheter tip location. Table 2 lists the location of the catheter tip under the fascia in the remaining 51 cases. There were no significant differences in the position of the catheter tip between the CFNB groups.
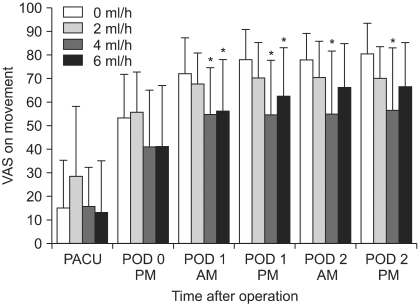
The decreased cumulative bolus use of IV PCA was observed in all CFNB groups compared to the control (0 ml/h, SFNB) beginning 6 PM on POD 1 through to 6 PM on POD 2, respectively (P < 0.05) (Fig. 3). The resting VAS scores were significantly lower in the 4 ml/h and 6 ml/h groups compared to the control on PODs 1 and 2, respectively, but only on POD 2 in the 2 ml/h group (P < 0.05) (Fig. 4). Lower VAS scores on movement were observed in the 4 ml/h group compared to the control on PODs 1 and 2, but only on POD 1 in the 6 ml/h group (P <0.05) (Fig. 5). Overall, the bolus use of IV PCA, and the VAS pain scores during rest and activity were similar in the groups on the day of surgery (Fig. 3-5).
Although, all CFNB groups used lower IV PCA doses than the control, it is believed that the minimum effective infusion rate of 0.125% bupivacaine would be 4 ml/h according to the VAS scores. Analgesia is not further improved by increasing the infusion rate to 6 ml/h. The infusion rate of 2 ml/h provided ineffective analgesia compared to the other two CFNB groups.
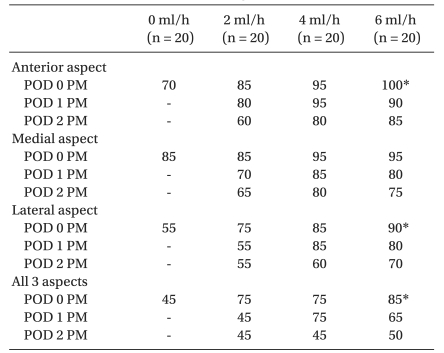
Table 3 shows sensory block on the anterior, medial, lateral and all 3 aspects of the thigh over time. The sensory block in the control (SFNB) was compared with those in the other three CFNB groups only at 6 PM on the day of surgery (POD 0), but not on PODs 1 and 2 because the sensory block in the control had mostly dissipated on PODs 1 and 2. There was a significant difference in sensory block on the anterior and lateral aspects of the thigh between the control and 6 ml/h groups at 6 PM on the day of surgery (P < 0.05). However, there was no significant difference in sensory block on each aspect of the thigh between the 2 ml/h and the other two CFNB groups during the study. In all 3 aspects of the thigh, there was a significant difference between control and 6 ml/h at 6 PM on the day of surgery (P < 0.05), but no significant difference between the 2 ml/h and other two CFNB groups during the study. The sensory block in the SFNB had mostly dissipated by 9 AM on POD 1. At 9 AM on POD 1, only 20% of patients in SFNB still showed sensory evidence of a FNB (data not shown) but no patient in SFNB showed any sensory evidence of a FNB by 6 PM on POD 1. The sensory block in all CFNB groups had dissipated over time. Although not verified, the regression of sensory block was faster in the 2 ml/h than the other two CFNB groups. No patient showed clinical evidence of local anesthetic toxicity.
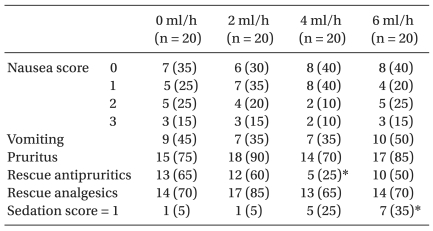
Overall, the incidence of PONV, pruritus and rescue analgesics were similar in the control and all CFNB groups (Table 4). The incidence of a sedation score = 1 was significantly more frequent in the 6 ml/h group than in the control group (P < 0.05) (Table 4). No patient had a sedation score ≥2, and no significant sedation was observed in any of the patients. There was no patient with respiratory rate <10 breaths/min. No patient required additional oxygen in the ward. Consequently, no patient showed evidence of respiratory depression.
To our knowledge, this is the first study in which the minimum effective infusion rate of 0.125% bupivacaine for CFNB in terms of the VAS pain scores appears to be 4 ml/h after TKR. We expected better analgesia with a high infusion rate of bupivacaine but analgesia was not improved further by increasing the infusion rate up to 6 ml/h. There may be two explanations for this observation. Firstly, there is no anatomical fascial sheath capable of conveying a local anesthetic solution or a catheter from below the inguinal ligament to the lumbar plexus [9]. Therefore, the spread of local anesthetic to the three nerves (the femoral, obturator, and lateral femoral cutaneous nerves) is difficult to achieve even in sufficient quantities. Secondly, the dose range of the infusion rate from 4 to 6 ml/ h used in this study was probably too small to allow any meaningful comparisons of the VAS pain scores. Van der Elst et al. [10] reported that a 3-in-1 block provides similar good pain relief with a continuous infusion rate of 10 ml/h to that of 15 ml/h after a total hip replacement. They further stated that neither the extent of anesthesia nor the quality of analgesia is improved by increasing the infusion rate or by adding PCA boluses. However, further studies will be needed to determine if there is any difference in analgesic efficacy of continuous femoral block between low (4 ml/h as in our study) and high (10 ml/h as in Van der Elst et al.'s study [10]) infusion rates of 0.125% bupivacaine. On the other hand, 2 ml/h provided inadequate pain relief compared to the other two CFNB groups. Hence, 2 ml/h is considered too low to spread under the fascia for a multiple nerve blockade.
Overall, the VAS pain scores during rest and activity, and the bolus use of IV PCA were similar in the groups on the day of surgery. This might be explained by the fact that significant analgesia might have been achieved in part with IT morphine. Therefore, no meaningful comparisons of the VAS pain scores and the bolus use of IV PCA in the groups could be obtained on the day of surgery. The other contributing factor would be that the SA provided preemptive analgesia [11]. Overall, all patients had excellent pain relief on the day of surgery.
Local anesthetic accumulation and toxicity is a potential problem with the continuous FNB technique. Esteve et al. [3] used a bolus dose of 2.5 mg/kg of 0.5% bupivacaine with epinephrine followed by the continuous infusion of 0.125% bupivacaine at 12 ml/h without epinephrine during 48 hours. In their study, the mean plasma bupivacaine level was 1.78 ± 0.59 µg/ml, and no neurological complications were recorded. The reported toxic concentration of bupivacaine is 2 µg/ml [12]. The bolus dose and infusion rate of 4 ml/h in the present study was one third of those reported by Esteve et al. Although the bupivacaine levels were not measured during CFNB, it is believed that a rate of 4 ml/h would be a safe dose in terms of local anesthetic toxicity.
The pain intensity and opioid consumption are routine measures of the outcome. However, opioid consumption probably is not a very reliable index for assessing the level of analgesia because there is no convincing evidence for proportionality between the postoperative pain intensity and analgesic requirements [13]. These results show that the cumulative bolus doses of IV PCA consumed in all CFNB groups were lower than that in the SFNB group. This suggests that all CFNB groups are more effective in analgesia than SFNB. However, considering the VAS, 4 ml/h appears to be superior to 2 ml/h, whereas 6 ml/h does not provide any added advantage. Therefore, a larger volume (6 ml/h) provides no additional benefit and a smaller volume (2 ml/h) is less effective. It is believed that the VAS scores would be more sensitive in capturing the differences in pain during periods of intense stimulation. The obvious downside to using morphine consumption as a marker of pain is that patients are unwilling to use of morphine due to nausea, or patients may use morphine for other reasons besides pain [14]. In the present study, some patients were concerned about the possible nausea and delayed wound healing because of the use of IV PCA, and were unwilling to demand a dose frequently enough to achieve satisfactory pain control. To review the data, the number of patients who were reluctant to use IV PCA because of nausea was 4 in 0 ml/h, 3 in 2 ml/h, 1 in 4 ml/h and none in 6 ml/h.
Overall, SFNB had greater VAS scores than the CFNB groups despite the higher cumulative bolus use of IV PCA than in the CFNB groups. It was believed that the higher cumulative bolus use of IV PCA may be associated with adequate pain relief in SFNB but this did not occur. It is possible that the use of IV PCA by the patient was not adequate or the bolus dose of 0.5 mg morphine was too small and an 8 min lockout was too long to alleviate severe surgical pain. The adequate demand dose of morphine for PCA is controversial. Owen et al. [15] indicated that a bolus dose size of 0.5 mg morphine was inadequate for many patients, whereas 2 mg was often too large, and a dose of 1 mg morphine was optimal. However, it was expected that the bolus dose of morphine could be reduced from 1 mg to 0.5 mg for adequate analgesia when combined with ketorolac. Indeed, Picard et al. [16] reported that a combination of half-doses of morphine (0.5 mg/ml) and ketorolac (1.5 mg/ml) is more effective in controlling postoperative pain than morphine (1 mg/ml) or ketorolac (3 mg/ml) alone.
As expected, a sensory block to a pinprick from SFNB had mostly dissipated by 9 AM on POD 1. However, severe postoperative pain after TKR persisted for more than 2 days [17], and SFNB is inadequate for pain relief after TKR. This is consistent with the findings of Fournier et al. [18] in which for a longer term pain relief, a single shot technique FNB appeared to be inadequate.
Three catheters showed coiling in the femoral head area, even though the length inserted had been 10-15 cm. Therefore, the length of insertion is a poor indicator of location of the catheter. In one patient who showed catheter-coiling with a failed block, the catheter had been inserted when a sartorius muscle twitch was elicited. In that patient, another femoral catheter was reinserted using a nerve stimulator in the PACU.
The distribution of the sensory block depends on the location of the catheter tip under the fascia iliaca [19]. A comparison of the different femoral infusion rates of bupivacaine administered by femoral catheters is possible only if the position of the catheter tip can be verified to avoid methodological bias in data analysis [19]. These clinical findings show that the course of the catheter under the fascia was unpredictable. Although only 51 patients were analyzed in this study, the location of the catheter under the fascia was similar in the infusion groups. Therefore, it is believed that the position of the catheter tip among the CFNB groups would not affect the results.
The method for assessing a sensory block in the cutaneous distribution of the obturator nerve is controversial. The cutaneous distribution of the obturator and femoral nerve in the medial aspect of the thigh cannot be distinguished by a pinprick test. Recently, Bouaziz et al. [20] reported that the only way to effectively evaluate the obturator nerve function is to assess the adductor strength. Furthermore, Macalou et al. [21] stated that an assessment of the obturator nerve block only based on a cutaneous blockade should be considered obsolete. Unfortunately, this study did not assess the adductor strength for an obturator nerve block. Therefore, Table 3 in this study presented a sensory block on the anterior, medial, and lateral aspects, instead of a sensory block in the cutaneous distribution of the femoral, obturator, and lateral femoral cutaneous nerves.
In this study, epinephrine 5 µg/ml (1 : 200,000) was added to 0.25% bupivacaine as a loading dose for FNB. Epinephrine is used with local anesthetics for two reasons. It slows the absorption of local anesthetic, thereby resulting in more profound analgesia with a longer duration. Perhaps more importantly, epinephrine, by reducing the rate of absorption, also reduces the blood levels of local anesthetic drugs [22]. Bupivacaine increases the skin blood flow after an intradermal injection in human skin, producing vasodilatation [23,24]. Epinephrine has a vasoconstrictor effect when coinjected with clinical doses of bupivacaine [24]. Therefore, it increases the duration of dermal analgesia of bupivacaine [23]. Epinephrine may also increase the sensory block and improve the painrelieving effect of a mixture of bupivacaine and fentanyl infused epidurally at a thoracic level after major thoracic or abdominal surgery [25]. In contrast, Misra et al. [26] demonstrated that the addition of epinephrine 5 µg/ml to bupivacaine did not affect significantly the duration of postoperative analgesia after combined femoral 3-in-1 and sciatic nerve blocks in patients undergoing TKR under SA. However, they did not assess the pain intensity at rest or on movement. Therefore, the quality of pain relief when adding epinephrine to bupivacaine could not be determined. To evaluate the time taken for return of sensation from femoral 3-in-1 block, they assessed the sensory block only at the lateral part of the thigh, not at the three aspects of thigh (the anterior, medial and lateral). Furthermore, they noted that some patients experienced pain at the site of the surgery before the complete return of sensation in the representative areas tested. They measured the mean time to first analgesia in all 22 patients but five of them received opioids for pain in areas other than the operated knee, thereby confounding the results. Therefore, further study is needed to evaluate the effect of epinephrine added to bupivacaine during femoral infusion to provide postoperative pain relief in patients undergoing TKR.
There were several limitations in this study. First, this study did not include patients who had received doses >6 ml/h of 0.125% bupivacaine. A future study will be needed to determine if there is any benefit of larger infusion rates compared to 4 ml/h. Secondly, the insertion of femoral catheters into patients in SFNB and the infusion of saline into the catheter, as would be required for a truly blinded study, were considered inappropriately invasive and unethical. Therefore, no sham catheter infusion was performed. Thirdly, in patients randomized to SFNB, the local anesthetic solution was injected immediately before the induction of SA, but was injected after surgery in those patients randomized to CFNB. The local anesthetic solution should have been injected after surgery in patients randomized to the control in the same manner as in those randomized to CFNB. This study suggested that performing a SFNB after surgery would have been more beneficial for prolonged postoperative analgesia. However, it was difficult to find the patella twitch with a nerve stimulator when there are extensive dressings around the knee after surgery. Finally, the incidence of a successful block in SFNB is unknown because the sensory function was not assessed before SA due to the busy operating room. This study relied on nerve stimulator guidance and evidence of muscle contraction to indicate the block accuracy.
In conclusion, the minimum effective infusion rate of 0.125% bupivacaine for CFNB after TKR would be 4 ml/h. Analgesia was not improved further by increasing the infusion rate to 6 ml/h.
References
1. Allen HW, Liu SS, Ware PD, Nairn CS, Owens BD. Peripheral nerve blocks improve analgesia after total knee replacement surgery. Anesth Analg. 1998; 87:93–97. PMID: 9661553.

2. Capdevila X, Barthelet Y, Biboulet P, Ryckwaert Y, Rubenovitch J, d'Athis F. Effects of perioperative analgesic technique on the surgical outcome and duration of rehabilitation after major knee surgery. Anesthesiology. 1999; 91:8–15. PMID: 10422923.

3. Esteve M, Veillette Y, Ecoffey C, Orhant EE. Continuous block of the femoral nerve after surgery of the knee: pharmacokinetics of bupivacaine. Ann Fr Anesth Reanim. 1990; 9:322–325. PMID: 2400142.
4. Hirst GC, Lang SA, Dust WN, Cassidy JD, Yip RW. Femoral nerve block: single injection versus continuous infusion for total knee arthroplasty. Reg Anesth. 1996; 21:292–297. PMID: 8837185.
5. Mansour NY, Bennetts FE. An observational study of combined continuous lumbar plexus and single-shot sciatic nerve blocks for post-knee surgery analgesia. Reg Anesth. 1996; 21:287–291. PMID: 8837184.
6. Marhofer P, Schrögendorfer K, Wallner T, Koinig H, Mayer N, Kapral S. Ultrasonographic guidance reduces the amount of local anesthetic for 3-in-1 blocks. Reg Anesth Pain Med. 1998; 23:584–588. PMID: 9840855.

7. Ng HP, Cheong KF, Lim A, Lim J, Puhaindran ME. Intraoperative single-shot "3-in-1" femoral nerve block with ropivacaine 0.25%, ropivacaine 0.5% or bupivacaine 0.25% provides comparable 48-hr analgesia after unilateral total knee replacement. Can J Anaesth. 2001; 48:1102–1108. PMID: 11744586.
8. Capdevila X, Biboulet P, Bouregba M, Barthelet Y, Rubenovitch J, d'Athis F. Comparison of the three-in-one and fascia lliaca compartment blocks in adults: clinical and radiographic analysis. Anesth Analg. 1998; 86:1039–1044. PMID: 9585293.
9. Dalens B, Tanguy A, Vanneuville G. Lumbar plexus blocks and lumbar plexus nerve blocks. Anesth Analg. 1989; 69:852–854. PMID: 2589668.

10. Van der Elst P, Singelyn FJ. Influence of different infusion rates on postoperative analgesic efficacy of continuous "3-in-1" block after total hip replacement. Anesthesiology. 1997; 87:A776.
11. Vaida SJ, Ben-David B, Somri M, Croitoru M, Sabo E, Gaitini L. The influence of preemptive spinal anesthesia on postoperative pain. J Clin Anesth. 2000; 12:374–377. PMID: 11025237.

12. Reynolds F. Comparison of the potential toxicity of bupivacaine, lignocaine and mepivacaine during epidural blockade for surgery. Br J Anaesth. 1971; 43:567–572. PMID: 5089935.
13. Kissin I. Preemptive analgesia: why its effect is not always obvious. Anesthesiology. 1996; 84:1015–1019. PMID: 8623993.
14. Sites BD, Beach M, Gallagher JD, Jarrett RA, Sparks MB, Lundberg CJ. A single injection ultrasound-assisted femoral nerve block provides side effect-sparing analgesia when compared with intrathecal morphine in patients undergoing total knee arthroplasty. Anesth Analg. 2004; 99:1539–1543. PMID: 15502061.

15. Owen H, Plummer JL, Armstrong I, Mather LE, Cousins MJ. Variables of patient-controlled analgesia. 1. Bolus size. Anaesthesia. 1989; 44:7–10. PMID: 2929911.

16. Picard P, Bazin JE, Conio N, Ruiz F, Schoeffler P. Ketorolac potentiates morphine in postoperative patient-controlled analgesia. Pain. 1997; 73:401–406. PMID: 9469531.

17. Park CK, Cho CK, Shin HH, Cho JH. The effect of intrathecal morphine added to continuous femoral 3-in-1 nerve block for analgesia after total knee replacement. Korean J Anesthesiol. 2008; 54:544–551.

18. Fournier R, Van Gessel E, Gaggero G, Boccovi S, Forster A, Gamulin Z. Postoperative analgesia with "3-in-1" femoral nerve block after prosthetic hip surgery. Can J Anaesth. 1998; 45:34–38. PMID: 9466024.

19. Capdevila X, Biboulet P, Morau D, Bernard N, Deschodt J, Lopez S, et al. Continuous three-in-one block for postoperative pain after lower limb orthopedic surgery: where do the catheters go? Anesth Analg. 2002; 94:1001–1006. PMID: 11916812.

20. Bouaziz H, Vial F, Jochum D, Macalou D, Heck M, Meuret P, et al. An evaluation of the cutaneous distribution after obturator nerve block. Anesth Analg. 2002; 94:445–449. PMID: 11812716.

21. Macalou D, Trueck S, Meuret P, Heck M, Vial F, Ouologuem S, et al. Postoperative analgesia after total knee replacement: the effect of an obturator nerve block added to the femoral 3-in-1 nerve block. Anesth Analg. 2004; 99:251–254. PMID: 15281539.

22. Moore DC, Scurlock JE. Possible role of epinephrine in prevention or correction of myocardial depression associated with bupivacaine. Anesth Analg. 1983; 62:450–453. PMID: 6829949.

23. Cederholm I, Akerman B, Evers H. Local analgesic and vascular effects of intradermal ropivacaine and bupivacaine in various concentrations with and without addition of adrenaline in man. Acta Anaesthesiol Scand. 1994; 38:322–327. PMID: 8067217.

24. Newton DJ, McLeod GA, Khan F, Belch JJ. The effect of adjuvant epinephrine concentration on the vasoactivity of the local anesthetics bupivacaine and levobupivacaine in human skin. Reg Anesth Pain Med. 2004; 29:307–311. PMID: 15305248.

25. Niemi G, Breivik H. Adrenaline markedly improves thoracic epidural analgesia produced by a low-dose infusion of bupivacaine, fentanyl and adrenaline after major surgery. A randomised, double-blind, cross-over study with and without adrenaline. Acta Anaesthesiol Scand. 1998; 42:897–909. PMID: 9773133.

26. Misra U, Pridie AK, McClymont C, Bower S. Plasma concentrations of bupivacaine following combined sciatic and femoral 3 in 1 nerve blocks in open knee surgery. Br J Anaesth. 1991; 66:310–313. PMID: 2015146.

Fig. 1
Frontal radiographs of the pelvic region show the location of the catheter tip (arrows) and the spread of the 3 ml of contrast media. (A) The tip is located under the iliac fascia covering the psoas major muscle (medial). (B) The tip of the catheter is located between the medial and lateral position (intermediate). (C) The tip is located under the iliac fascia covering the iliacus muscle (lateral).
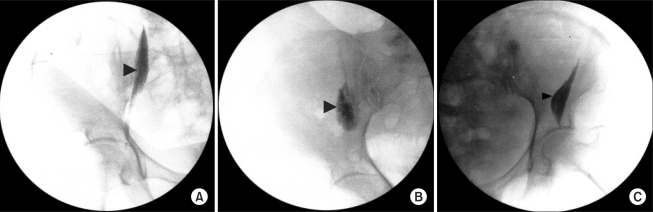
Fig. 2
This figure shows the zones reached by the spread of the local anesthetic under the fascia iliaca. (A) Internal spread under the psoas muscle fascia. (B) External spread under the iliacus muscle fascia. (C) Spread to the roots of the lumbar plexus. 1 = lateral femoral cutaneous nerve, 2 = femoral nerve, 3 = obturator nerve (Reprinted from Capdevila et al.'s study [8]).
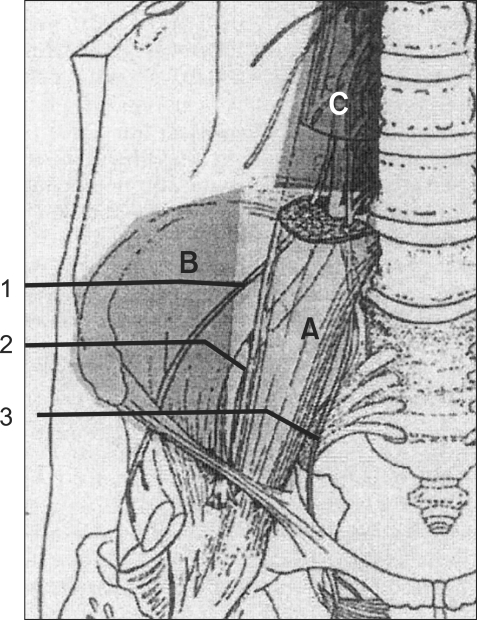
Fig. 3
This diagram compares the cumulative postoperative consumption of IV PCA (ml) by patients in the four groups during the first postoperative 2 days. All CFNB groups show lower cumulative PCA bolus use of morphine plus ketorolac than the control on PODs 1 and 2, respectively. PCA: patient-controlled analgesia, CFNB: continuous femoral nerve block, PACU: postanesthesia care unit, POD: postoperative day. *P < 0.05 compared to the 0 ml/h group.
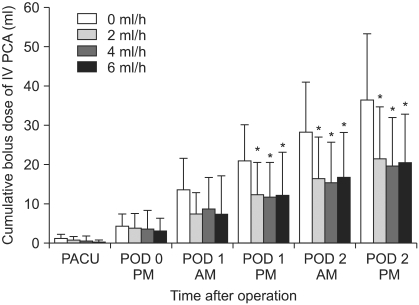
Fig. 4
This diagram compares the VAS pain scores at rest in the four groups. The VAS scores at rest are significantly lower in the 4 ml/h and 6 ml/h groups than the control on PODs 1 and 2, respectively, but only on POD 2 in 2 ml/h. VAS: visual analog scale, PACU: postanesthesia care unit, POD: postoperative day. *P < 0.05 compared to the 0 ml/h group.
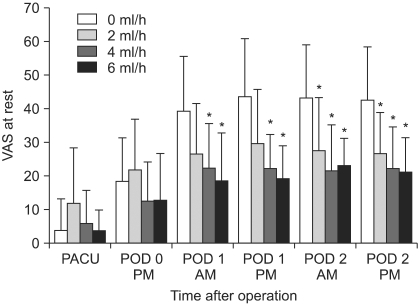
Fig. 5
This diagram compares the VAS pain scores on movement in the four groups. The VAS scores on movement in the 4 ml/h group are significantly lower than the control on PODs 1 and 2, but only on POD 1 in 6 ml/h. VAS: visual analog scale, PACU: postanesthesia care unit, POD: postoperative day. *P < 0.05 compared to the 0 ml/h group.




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download