Introduction
Reference models for zero interaction
Bliss independence

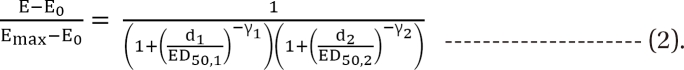
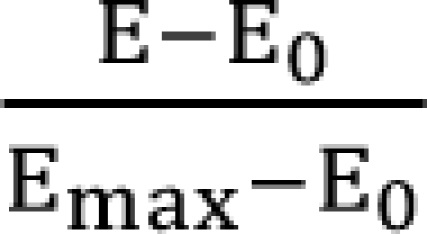 is a fraction of the effect resulting from the mixture of d1 and d2; d1 and d2 are doses of each drug in the mixture that yield the effect E; ED50,1 or 2 is a dose of drugs to produce 50% of the maximal effect (Emax) for each drug acting alone; and γ1 and γ2 are Hill coefficients (slope of dose-response curve). When Eq. (1) and (2) are recast in terms of the fraction of effect affected (fa1 or 2) (if 1 - fa exchanges fu in Eq. (1), because fa + fu = 1), then Eq. (3) and (4) are the results.
is a fraction of the effect resulting from the mixture of d1 and d2; d1 and d2 are doses of each drug in the mixture that yield the effect E; ED50,1 or 2 is a dose of drugs to produce 50% of the maximal effect (Emax) for each drug acting alone; and γ1 and γ2 are Hill coefficients (slope of dose-response curve). When Eq. (1) and (2) are recast in terms of the fraction of effect affected (fa1 or 2) (if 1 - fa exchanges fu in Eq. (1), because fa + fu = 1), then Eq. (3) and (4) are the results.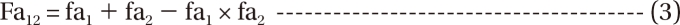
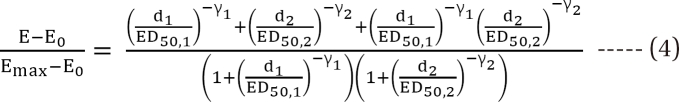
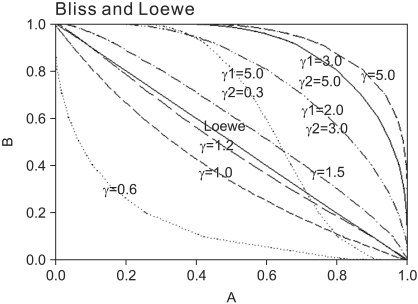 | Fig. 1Normalized isoboles at 50% effect level, for the Bliss independence model, Eq. (2), for various values of γ1 and γ2, which are listed next to each corresponding curve. A single γ indicates that γ1 = γ2 = γ. When Hill coefficients are different (one larger than) 1.3 and the other less than 1.2), the isoboles are S-shaped and may cross the Loewe additivity diagonal line. The solid diagonal line is The Loewe additivity line (Eq. (5)). The isobole for zero interaction of a mutually nonexclusive model (Eq. (9)) is the same curve as that of the Bliss independence model with γ = 1.0. |
Loewe additivity
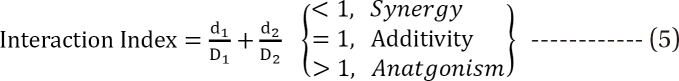
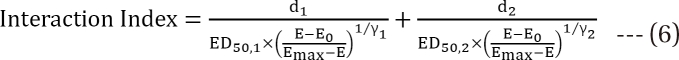
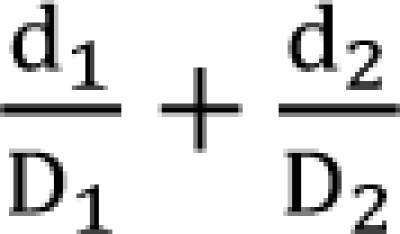 is called the interaction index at the combination dose (d1, d2). The dose (D1, or D2) for each single drug producing the same effect of E is expressed as
is called the interaction index at the combination dose (d1, d2). The dose (D1, or D2) for each single drug producing the same effect of E is expressed as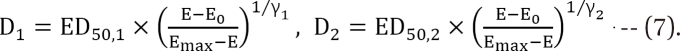
Median-effect method from the law of mass action
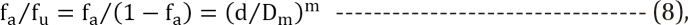
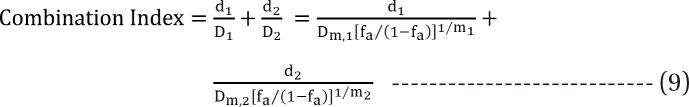
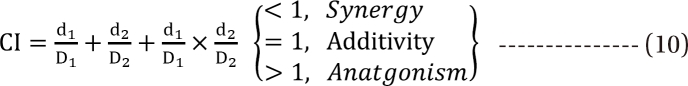
Response surface
Response surface models with a single interaction parameter
Greco model [34]
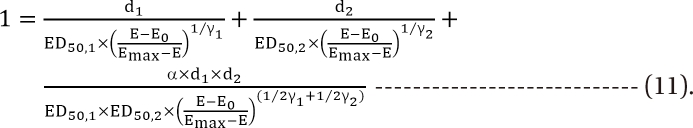
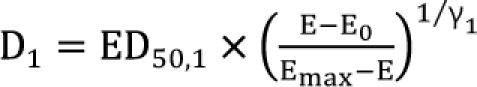 ,
, 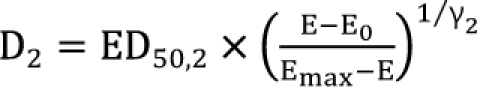 , the first two terms on the right-hand expression of Eq. (11) are equivalent to Eq. (5). Thus, Eq. (12) defines interaction index for two-drug combination.
, the first two terms on the right-hand expression of Eq. (11) are equivalent to Eq. (5). Thus, Eq. (12) defines interaction index for two-drug combination.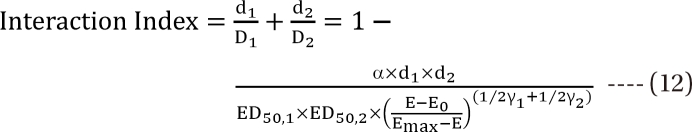
Machado model
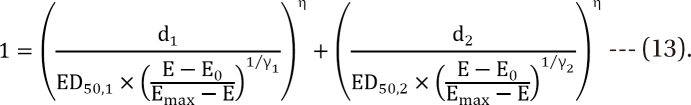
Plummer model
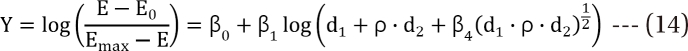
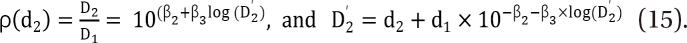
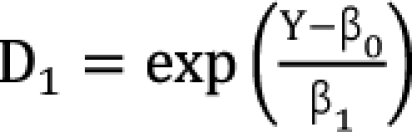 ,
, 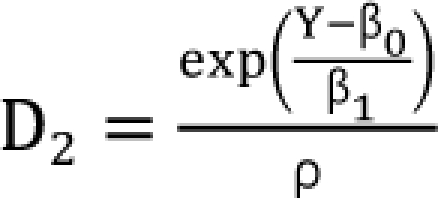 which are the doses of drug 1 and drug 2 producing the effect E. You can rearrange Eq. (14) to be
which are the doses of drug 1 and drug 2 producing the effect E. You can rearrange Eq. (14) to be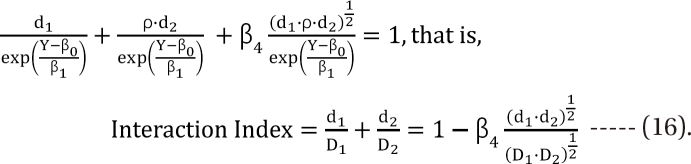
Carter model
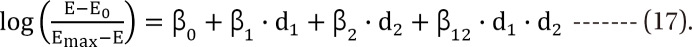
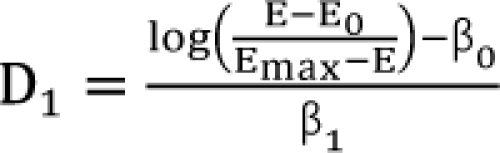 ,
, 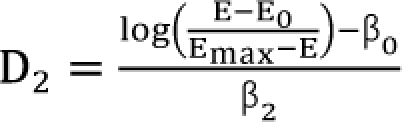 . After moving β0 to the left side of Eq. (17), and then dividing both sides of the equation with
. After moving β0 to the left side of Eq. (17), and then dividing both sides of the equation with 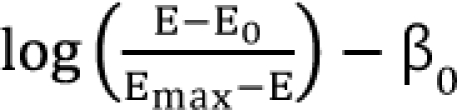 , you will obtain the interaction index:
, you will obtain the interaction index: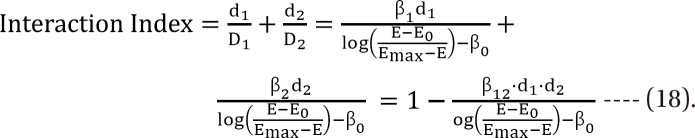
Response surface models with an interaction function
Minto model
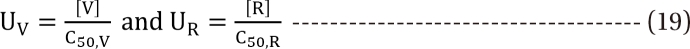
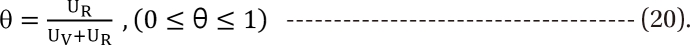
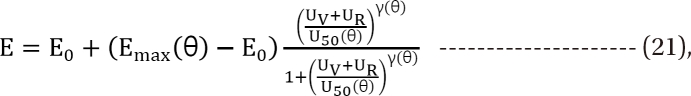
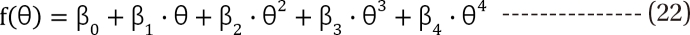
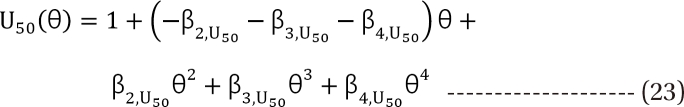
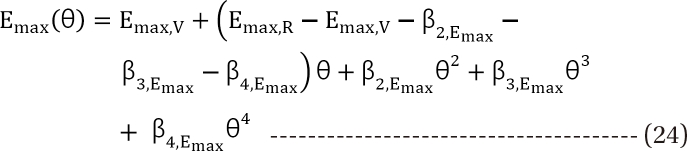
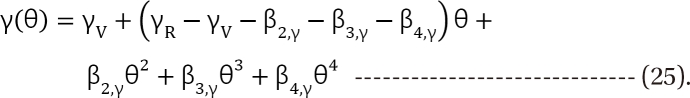
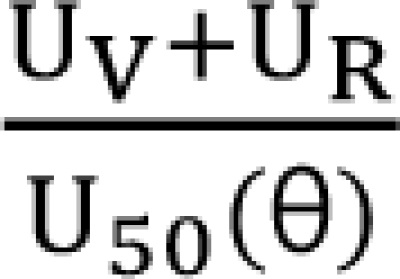 in Eq. (21). This will create a synergistic effect. If U50(θ) is greater than 1 for all values of θ, this lessen the term
in Eq. (21). This will create a synergistic effect. If U50(θ) is greater than 1 for all values of θ, this lessen the term 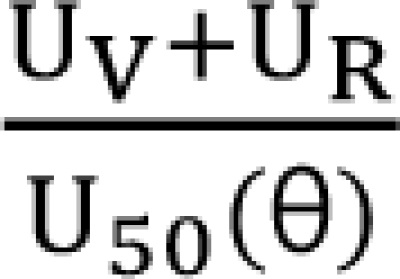 in Eq. (21). This will create an antagonistic effect. This model can be used in investigating the drug interaction when the maximum effects of drugs V and R are not identical.
in Eq. (21). This will create an antagonistic effect. This model can be used in investigating the drug interaction when the maximum effects of drugs V and R are not identical.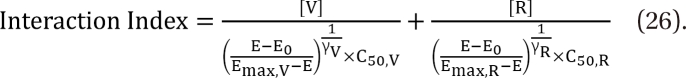
Fidler model
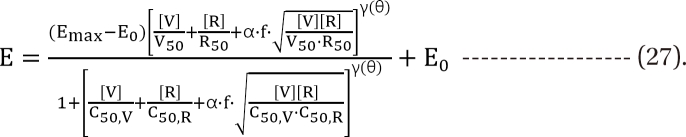
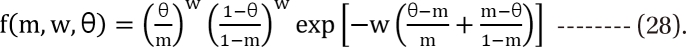
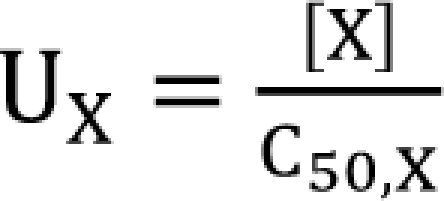 ,
, 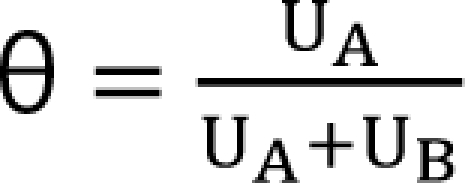 . The m term is the symmetry parameter of the potency ratio θ, where the maximum interaction occurs. The interaction scope parameter w specifies the uniformity of the interaction across various drug ratios. The parameters γ(θ) and Emax(θ) are given as functions of potency fraction:
. The m term is the symmetry parameter of the potency ratio θ, where the maximum interaction occurs. The interaction scope parameter w specifies the uniformity of the interaction across various drug ratios. The parameters γ(θ) and Emax(θ) are given as functions of potency fraction: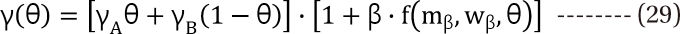
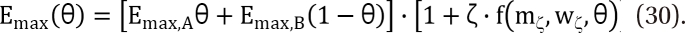
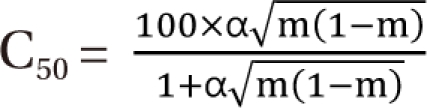 ) expresses % change of the 50% effect when compared with each drug in combination acting alone. Another feature is for the Fidler model to predict the concentration ratio of two drugs at the most effective points. In an asymmetric case, the best ratio is C50,V × m : C50,R × (1 - m); in a symmetric case, the best ratio is C50,V : C50,R.
) expresses % change of the 50% effect when compared with each drug in combination acting alone. Another feature is for the Fidler model to predict the concentration ratio of two drugs at the most effective points. In an asymmetric case, the best ratio is C50,V × m : C50,R × (1 - m); in a symmetric case, the best ratio is C50,V : C50,R.Kong model
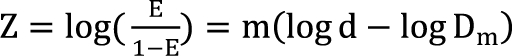 . It is assumed that the logit-transformed response follows a linear function of log (d) for each of two drugs when acting independently. The dose-response curve denotes
. It is assumed that the logit-transformed response follows a linear function of log (d) for each of two drugs when acting independently. The dose-response curve denotes 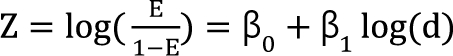 . The model proposed by Kong and Lee has a square root term and the relative potency. So, it can be regarded as a generalization of Finney's model [33] and the model derived by Plummer and Short [36]. To construct a generalized model, including the nonparallel dose-response curves of two drugs, a varying relative potency should be allowed. When the two drugs have the nonparallel dose-response curves, or different slopes, each drug combination ([V], [R]) on the z-isobole shares the same relative potency ρ(z), and its equivalent amount dose is [V] + ρ(z) [R] in terms of drug V, or ρ(z)-1 [V] + [R] in terms of drug R. That is, the combination doses on different isoboles may have different relative potencies. The form of ρ(z) is derived as
. The model proposed by Kong and Lee has a square root term and the relative potency. So, it can be regarded as a generalization of Finney's model [33] and the model derived by Plummer and Short [36]. To construct a generalized model, including the nonparallel dose-response curves of two drugs, a varying relative potency should be allowed. When the two drugs have the nonparallel dose-response curves, or different slopes, each drug combination ([V], [R]) on the z-isobole shares the same relative potency ρ(z), and its equivalent amount dose is [V] + ρ(z) [R] in terms of drug V, or ρ(z)-1 [V] + [R] in terms of drug R. That is, the combination doses on different isoboles may have different relative potencies. The form of ρ(z) is derived as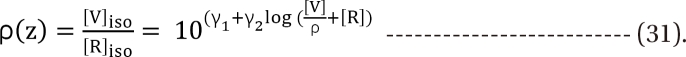
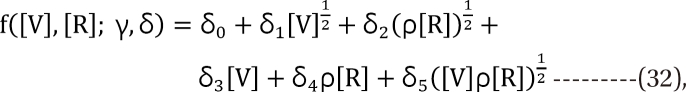
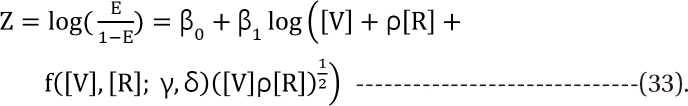
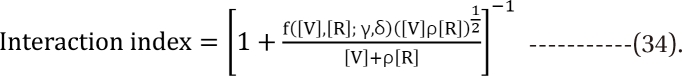
Data analysis
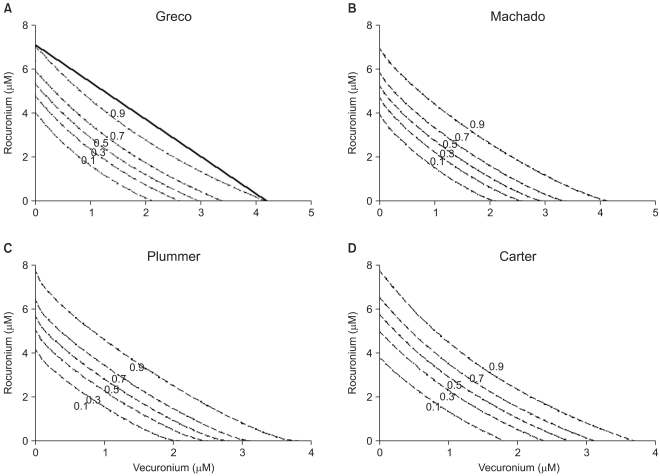 | Fig. 2The Greco model (A), the Machado model (B), the Plummer model (C), and the Carter model (D) show isoboles of the response surface. The abscissa is a concentration of vecuronium (µM) and the ordinate, that of rocuronium (µM). The numbers in the plots are the fractional increase of tetanic fade. The thick, solid line in panel A is shown as an example of the Loewe additivity line at a 0.9 effect. If all the Loewe additivity lines would be drawn, these would reveal that the isoboles are upward concave. Therefore, it is concluded that interactions between vecuronium and rocuronium are consistently synergistic. |
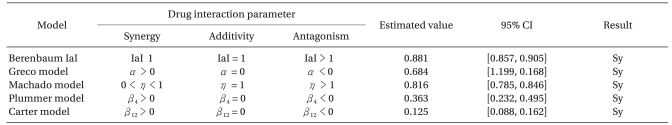
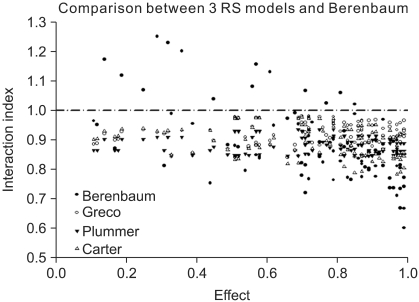 | Fig. 3The plot of interaction indices of actual data points was drawn. The interaction index of the Machado model cannot be calculated due to the limitation of the model. Four models, except for the Berenbaum method, consistently have interaction indices of less than 1, which means synergistic interaction. The Berenbaum method reveals that most interaction indices are less than 1 at a high effect region, and are greater than 1 at a low effect region. According to the Berenbaum method, combinations of the two drugs have all types of interaction. Therefore, the response surface models with a single interaction parameter are inadequate in analyzing data which has a complicated scatter of different types of drug interactions. |
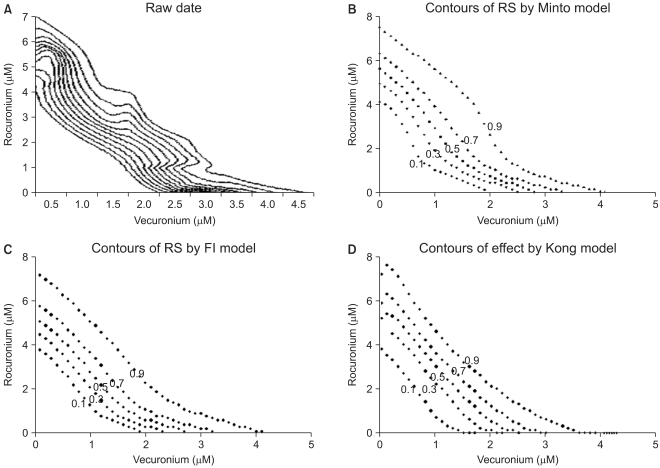 | Fig. 4This figure shows isoboles of the response surface derived from raw data (A), Minto model (B), Fidler model (C), and Kong model (D). The isoboles of raw data (A) are rendered via the Renka I (nonparametric algorithm) procedure. The abscissa is a concentration of vecuronium (µM) and the ordinate, that of rocuronium (µM). The numbers in the plots are the fractional increase of tetanic fade. If the Loewe additivity line would be drawn, this would reveal what type of drug interaction the isobole has. |
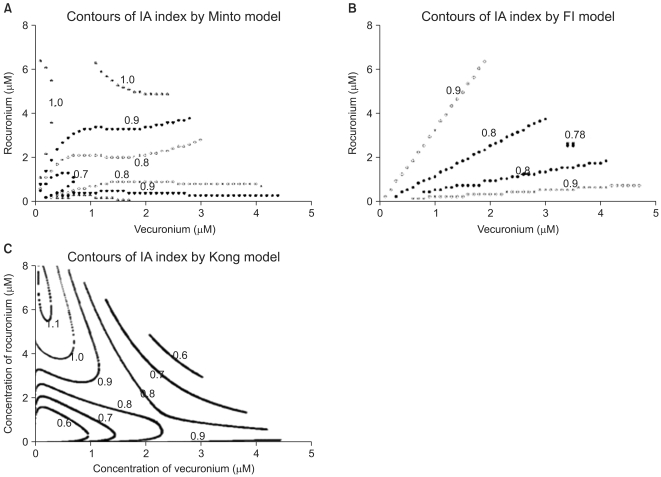 | Fig. 5The contours of the interaction index calculated based on the Minto model (A), Fidler model (B), and Kong model (C) are plotted. The value "1.0" in the contour plot of the interaction indices represents additivity; the values less than 1.0 represent synergistic interaction; and the ones more than 1.0 represent antagonistic interaction. Chou and Hayball proposed that an interaction index from 0.9 to 1.1 is designated as being additive. Based on the Minto and Fidler models, the interactions of the two drugs are synergistic and additive. By the Kong model, the interactions of the two drugs are synergistic, additive, and antagonistic. The interspersion of the interaction indices on the entire surface is relatively different among three models. The abscissa is a concentration of vecuronium (µM) and the ordinate, that of rocuronium (µM). |
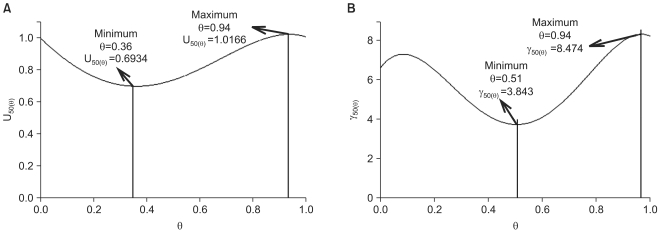 | Fig. 6The functions for the parameters U50(θ), and γ(θ) for the two drug interaction show a synergistic interaction (U50(θ)=0.6934) at θ=0.36, which is the ratio of the potency unit of 0.64 : 0.36, vecuronium : rocuronium (A), and varying steepness depending on the unique ratio of two drugs (B). |
Table 2
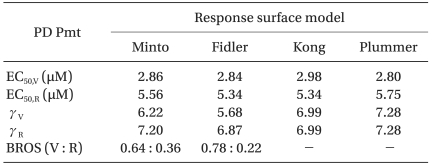
BROS (V : R): best ratio of synergy (vecuronium : rocuronium), EC50,V and EC50,R: median effect of vecuronium and rocuronium, respectively, γV and γR : Hill slope of vecuronium and rocuronium, PD Pmt: pharmacodynamic parameter, RS: response surface, Kong and Plummer: assume that relative potency ratio is constant (or the dose-effect curves are parallel).




 PDF
PDF Citation
Citation Print
Print


 XML Download
XML Download