Abstract
Pneumothorax associated with a pneumoperitonium in laparoscopic surgery is rare but can cause life-threatening complications. A 62-year-old man was scheduled for a laparoscopy-assisted Billroth-I gastrectomy under general anesthesia. Approximately 70 minutes after insufflating carbon dioxide into the intraabdominal cavity at a pressure of 12 mmHg, the peak inspiratory pressure increased, while the oxygen saturation decreased. The pneumothorax of the left lung was evident on the intraoperative chest radiograph. The pneumothorax improved after inserting a catheter into the affected area. The cause of the pneumothorax was unknown but an anatomical defect is believed responsible. This report shows that pneumothorax developed under an intraabdominal pressure in the conventional safety range. Careful monitoring and immediate treatment is necessary to prevent the condition from worsening.
Pneumothorax during laparoscopic surgery using a pneumoperitonium quite rare but can cause life-threatening complications if immediate and proper treatment is not provided [1-5]. Pneumothorax during laparoscopy developed as a result of iatrogenic damage to the diaphragm or hiatal hernia and a rupture in the alveolus. Some cases so not show definite causes, such as congenital defects in the diaphragm, immaturity of the membranes between the thoracic cavity and abdominal cavity, or gas (air) leaking through the retroperitoneal cavity, etc [1,6]. Generally, factors, such as the type of surgery, prolonged time of the pneumoperitonium, high gas pressurizing pressure in the abdominal cavity, etc. are believed to influence the development of pneumothorax [7-9].
The authors report their experience of spontaneous pneumothorax during laparoscopic distal gastrectomy with a relatively low intraperitoneal gas pressuring pressure without any significant damage with a review of the relevant literature.
A 62-year-old man, 170 cm, 72 kg, with early gastric cancer diagnosed during a medical inspection 1 month earlier was admitted to hospital for a laparoscopic gastrectomy. There was nothing in his medical record except for having taken medication for hypertension that was found in a medical inspection. A preoperative chest X-ray, pulmonary function test and electrocardiogram showed normal findings. Premedication was not administered, and the vital signs measured upon arrival showed a blood pressure (BP), heart rate (HR) and saturation of peripheral oxygen (SpO2) of 140/90 mmHg, 65/min and 99%, respectively. Fentanyl 0.1 mg and thiopental 325 mg were administered with 100% inspired oxygen through the anesthetic mask. After confirming a loss of consciousness, endotracheal intubation was performed after an intravenous injection of succinylcholine 70 mg.
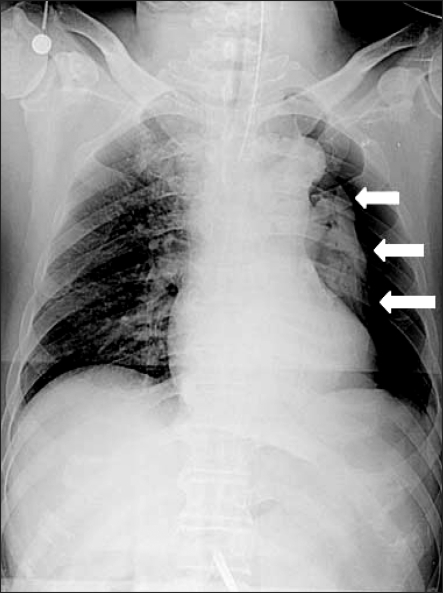
There were no problems with the intubation. After ventilation of both lungs had been confirmed, the tube was fixed to the incisal tooth at a depth of 23 cm, and vecuronium 6 mg was administered for muscle relaxation. A urinary catheter was inserted. Arterial puncturing was performed at the left radial artery for invasive monitoring of the arterial blood pressure. Venous catheterization was performed through the right external jugular vein. Anesthesia was maintained with oxygen 1.5 L/min, air 1.5 L/min, and sevoflurane 1.5-3.0 vol%, while the muscle relaxant was administered intermittently. Mechanical ventilation was performed with a tidal volume (TV) respiratory rate (RR) and peak airway pressure (Pmax) of 500 ml, 12/min, and 16 cmH2O, respectively. After inducing anesthesia, the partial end-tidal carbon dioxide pressure (PETCO2), BP, HR and SpO2 was 37 mmHg, 130/90 mmHg, 80/min and 99%, respectively. For laparoscopy, a total of 5 trocars (3 of 5 mm, 11 mm, and 12 mm) were inserted and an 11 mm Hasson trocar was connected to a carbon dioxide gas pump (CO2 endoflator, Olympus, Japan). To secure the operative view and space, 12 mmHg of CO2 was provided and the patient was turned to the reverse trendelenburg position, and the operation proceeded. Auscultation on both lungs after repositioning showed regular breathing sounds. The Pmax after the pneumoperitonium was 22 cmH2O, and the PETCO2 was 38 mmHg. Approximately 70 minutes after the pneumoperitonium, the Pmax increased suddenly to 30 cmH2O, and then endotracheal suction was performed to exclude the effect of endotracheal excretion but the amount excreted was small. Subsequently, the Pmax was maintained at the higher 30 cmH2O, and the SpO2 decreased to 96%. His vital signs then showed a PETCO2, BP, and HR of 39 mmHg, 110/60 mmHg and 75/min, respectively, while the arterial blood gas analysis (ABGA) indicated a pH, partial pressure of arterial carbon dioxide (PaCO2) and PaO2 of 7.37, 48 mmHg and 118 mmHg, respectively. Upon auscultation, the breathing sound of the right side was normal but the breathing sound of the left lung decreased significantly. Manual ventilation was performed to preclude the tube from moving into the bronchus and the endotracheal tube was withdrawn back to 4 cm with auscultation. However, the breathing sound of the left lung still decreased. There were no significant changes in the patient's BP and HR, but the friction of inspired oxygen (FiO2) was increased and another ABGA was used under consideration of the incidence of pneumothorax. The results indicated a pH, PaCO2 and PaO2 of 7.33, 53 mmHg and of 105 mmHg, respectively. The pneumoperitonium was terminated immediately and a chest X-ray (chest AP view) was performed. The findings on the chest x-ray confirmed the incidence of pneumothorax in the left lung (Fig. 1), and 12-French catheter (Trocar catheter, Mallinckrodt medical Inc, Ireland) was inserted along the midaxillary line into the space between the 5th and 6th rib after consulting with the surgeon. After catheterization, the Pmax was decreased to 24 cmH2O and the breathing sound of the left lung showed a favorable change. The PETCO2 was 38 mmHg, and the SpO2 increased to 99%. The ABGA findings indicated a pH of 7.37, a PaCO2 of 44 mmHg, and a PaO2 of 158 mmHg, indicating significant improvement. After confirming the stability in the patient and performing a pneumoperitonium with the same gas pressuring pressure for the operation, the FiO2 was reduced to 0.6 and mechanical ventilation was performed with a TV of 500 ml and RR of 14/min. The measured ABGA showed a pH, PaCO2 and PaO2 of 7.39, 44 mmHg, 192 mmHg, respectively, whereas the Pmax and PETCO2 was 24 cmH2O and 32 mmHg, respectively. The total surgical time was 4 hours.
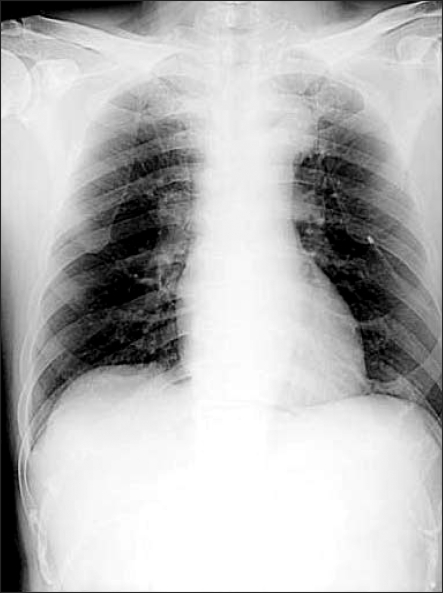
After his spontaneous respiration returned, muscle relaxation was achieved using a mixture of glycopyrrolate 0.4 mg and neostigmine 2 mg, and the endotracheal tube was removed after he reached consciousness. The patient did not complain of any discomfort or respiratory disturbance except for surgical site pain, and he was transported to the post-anesthesia recovery unit. Oxygen at 5 L/min was administered through a venturi mask when the pulse oximetry indicated a SpO2 of 99%. After the patient's consciousness returned to normal, he was transferred to the ward while oxygen of 3 L/min was provided through a venturi mask. A chest X-ray performed on the chest PA view confirmed a considerable decrease in the pneumothorax (Fig. 2). During his hospitalization, the patient did not complain of any discomfort of dyspnea. On postoperative day 4, the catheter was removed, and on postoperative day 6, a chest X-ray and computerized tomography (CT) did not show any abnormal findings. The patient was discharged home in good condition.
Pneumoperitonium, which is employed as a key step during a laparoscopy, can reduce the patient's residual volume, cause a high airway pressure and hypercapnia, as well as hemodynamic changes to senior patients with underlying disorders in the cardiovascular system [10]. Sometimes, pneumoperitonium not only affects the pulmonary function caused by the abdominal pressure, but also causes pneumothorax due to air flow into the thoracic cavity. The causes of pneumothorax during laparoscopy can be divided into cases in whom organic damage can and cannot be identified. The causes for pneumothorax with organic damage involves iatrogenic damage on the pleura, diaphragm, or hiatal hernia, and rupture in the alveolus, particularly which can be triggered by the presence of blebs or bullae in the lung, or by the incidence of barotraumas during mechanical ventilation. The following events are suspected to be the cause of pneumothorax without organic damage: the remains of congenital deficits between the abdominal cavity, pleura, and pericardium; movement of gas into the chest septum or the pleural cavity through the retroperitoneal space; gas leakage by a high gas pressuring pressure; and a long length of surgery time on vulnerable spots in the aortic or esophageal hiatus.
Regardless of the underlying cause, the symptoms of pneumothorax during laparoscopy vary from a minor case to serious ones in terms of the clinical features. A review of the literature also revealed a particular case, in which an operation encountered complete unilateral pulmonary collapse but with no increase in Pmax, nor any significant clinical signs on PETCO2 and SpO2, so the surgery proceeded uneventfully [11]. Such diversity in the symptoms of pneumothorax comes is due to the influence on the cardiovascular system being dependent on the size and developmental rate of the pneumothorax, as well as the existent cardiopulmonary function of the patients. Hence, the symptoms of the cases with organic damage are expected to be more rapid and severe. Therefore, an immediate diagnosis and proper treatment by the anesthesiologist is critical for the safety of patients. In this case, we detected abnormal findings by the increase in Pmax and decrease in SpO2 at the beginning of the procedure, and confirmed the pneumothorax by a chest X-ray while excluding an obstruction of endotracheal tube due to excretions, patient's spontaneous respiration, and migration of the tube into the bronchus. A study result, where changes in the QRS wave (detector describing the electrocardiographic features) of the precordial electrocardiogram recognize the incidence of an artificial pneumothorax in experiments, would be also useful for an earlier diagnosis of pneumothorax [12]. However, there are no clinical findings yet, and this case did not show such changes in the QRS wave.
Pneumothorax has a high incidence in cases of a long operating time of 200 minutes or more, when >15 mmHg on the pneumoperitonium is maintained, a PETCO2 >50 mmHg, and operations such as fundoplasty or hiatal dissection, etc. [2,11,13]. In this case, the operation proceeded with a Pmax of 12 mmHg, which was reported to minimize the hemodynamic changes, such as a decrease in cardiac output [14], instead of a Pmax of 10 mmHg reported in the literature in order to be free of any complications caused by the pneumoperitonium [9]. During the operation, we encountered the pneumothorax despite a PETCO2 of 40 mmHg or less and a short duration of the pneumoperitonium, 70 min. Based on the fact that after a postoperative CT on the chest, there were no findings of underlying disorders in the lung, and that the intraoperative observations and post-operative video reconfirmation did not reveal any iatrogenic damage nor exposure of the retroperitoneal space, we suspected air intrapleural influx through a congenital deficiency, such as a peripheral fragile region of the pleura, or a hiatus between the pleura and the peritoneum. Among the 220 cases of laparoscopic surgery performed at our institution during the last three years that had a Pmax of 15 mmHg or more and an operating time of 3 hours or more, there have not been any incidences of severe pneumothorax requiring air release, as in this case. Therefore, the presence of anatomical defect is considered to be a more important factor in the occurrence of pneumothorax than any other factors, which will need further data and research. In addition, the use of a relatively low gas pressuring pressure, such as that used in our case, would help relieve the cardiovascular suppression, but it cannot totally reduce the incidence of pulmonary complications, such as pneumothorax.
In most cases of pneumothorax, it is a primary step to resolve pneumoperitonium. When a patient returns to a stable condition, the operation should proceed with a lower gas pressuring pressure. However, decompression through positive end-expiratory pressure (PEEP) or thoracentesis should be attempted when such a reduction of the pressure fails to bring the patient back to the stable condition. In emergent cases, consideration would be given to a thoracostomy and conversion to a laparotomy [7]. In this case, the chest X-ray revealed complete collapse in the left lung. Considering the residual surgical time to be relatively long, catheterization was performed to reduce the pressure instead of PEEP. The operation proceeded after confirming the breathing sound on auscultation and improvement in ABGA.
In conclusion, anesthesiologists should always be aware of the incidence of respiratory complications during a laparoscopy, and they should undergo anesthetic management not only of the extent of gas pressuring pressure, duration of surgery, or presence of organic damage, but also be on guard against pneumothorax due to a possible anatomical defect.
References
1. Waterman BJ, Robinson BC, Snow BW, Cartwright PC, Hamilton BD, Grasso M. Pneumothorax in pediatric patients after urological laparoscopic surgery: experience with 4 patients. J Urol. 2004; 171:1256–1258. PMID: 14767324.

2. Murdock CM, Wolff AJ, Van Geem T. Risk factors for hypercarbia, subcutaneous emphysema, pneumothorax, and pneumomediastinum during laparoscopy. Obstet Gynecol. 2000; 95:704–709. PMID: 10775733.

3. Gabbott DA, Dunkley AB, Roberts FL. Carbon dioxide pneumothorax occurring during laparoscopic cholecystectomy. Anaesthesia. 1992; 47:587–588. PMID: 1385681.

4. Prystowsky JB, Jericho BG, Epstein HM. Spontaneous bilateral pneumothorax--complication of laparoscopic cholecystectomy. Surgery. 1993; 114:988–992. PMID: 8236025.
5. Togal T, Gulhas N, Cicek M, Teksan H, Ersoy O. Carbon dioxide pneumothorax during laparoscopic surgery. Surg Endosc. 2002; 16:1242. PMID: 12042908.

6. Shim HS, Park SH, Ryu DH, Kim IK, Shim MK. Pneumothorax during retroperitoneal laparoscopic nephrectomy. Korean J Anesthesiol. 2005; 48:324–327.
7. Mehran A, Brasesco O, De Velasco E, Szomstein S, Rosenthal R. Intra-operative pneumothorax complicating laparoscopic Rouxen-Y gastric bypass. Obes Surg. 2004; 14:124–128. PMID: 14980047.

8. Voyles CR, Madden B. The "floppy diaphragm" sign with laparoscopic-associated pneumothorax. JSLS. 1998; 2:71–73. PMID: 9876715.
9. Ferzli GS, Kiel T, Hurwitz JB, Davidson P, Piperno B, Fiorillo MA, et al. Pneumothorax as a complication of laparoscopic inguinal hernia repair. Surg Endosc. 1997; 11:152–153. PMID: 9069149.

10. Bardoczky GI, Engelman E, Levarlet M, Simon P. Ventilatory effects of pneumoperitoneum monitored with continuous spirometry. Anaesthesia. 1993; 48:309–311. PMID: 8494131.

11. Hawasli A. Spontaneous resolution of massive laparoscopy-associated pneumothorax: the case of the bulging diaphragm and review of the literature. J Laparoendosc Adv Surg Tech A. 2002; 12:77–82. PMID: 11905868.

12. Ludemann R, Krysztopik R, Jamieson GG, Watson DI. Pneumothorax during laparoscopy. Surg Endosc. 2003; 17:1985–1989. PMID: 14569446.

13. Song SO. Anesthetic management for laparoscopic cholecystectomy. Korean J Anesthesiol. 2004; 47:1–11.

14. Ishizaki Y, Bandai Y, Shimomura K, Abe H, Ohtomo Y, Idezuki Y. Safe intraabdominal pressure of carbon dioxide pneumoperitoneum during laparoscopic surgery. Surgery. 1993; 114:549–554. PMID: 8367810.




 PDF
PDF Citation
Citation Print
Print


 XML Download
XML Download