Abstract
Background
Propofol may decrease myocardial contractility via actions on the β-adrenoceptor-mediated signal transduction. The aim of this study was to evaluate the effect of propofol via β-adrenoceptor-mediated signal transduction by measuring the tissue levels of cAMP (cyclic adenosine monophosphate).
Methods
The effects of propofol on β-adrenoceptor mediated cascades were measured with cAMP concentrations, which were stimulated by agonists (l-isoproterenol, GTPγS, and forskolin) of each step of β-adrenoceptor-mediated cascades.
Propofol is a widely used intravenous anesthetic with advantageous properties such as rapid induction and emergence. However, induction of anesthesia with propofol is often associated with a decrease in systemic arterial pressure.
At supraclinical concentrations which may occur during rapid bolus injection, a direct inhibitory effect of propofol on myocardial contraction in normal cardiac tissues of guinea pig [1-3] and ferret [4] as well as human atrial muscle [5] has been reported. Contributing factors on myocardial depression by propofol such as decreased ICa (L-type Ca2+ current) via sarcolemma [6-8] and decreased Ca2+ uptake by the sarcoplasmic reticulum [9-11] have been proposed. Recently, propofol has been reported to have direct inhibitory effects on β-adrenoceptor signal transduction, which may cause contractile depression in cardiac muscle [12]. However, the cellular mechanism has not been well-defined.
β-adrenoceptor stimulated by l-isoproterenol binds to β-adrenergic receptor and produce an interaction between β-adrenergic receptor and Gs (stimulatory G-protein), which enhances adenylyl cyclase activity. Adenylyl cyclase is a membrane-bound enzyme that converts intracellular adenosine phosphate (ATP) to cAMP, a second messenger in the signal transduction system. cAMP activates cAMP-dependent protein kinase (A-kinase), resulting in phosphorylation of particular proteins. Increased cAMP prolongs the opening time of Ca2+ channels, resulting in increased Ca2+ entry and positive inotropic effect. Inhibitory effects on the signal transduction pathway may also result in negative inotropic effect.
Therefore, we evaluated the effect of propofol on tissue cAMP levels via β-adrenoceptor-mediated signal transduction using β-adrenoceptor agonist, G-protein stimulant, and adenylyl cyclase agonist.
The heart was excised from male guinea pigs (250-300 g) anesthetized with sevoflurane at 3-4 vol% according to a procedure approved by the Institutional Animal Research Committee. Blood was evacuated and the hearts were kept in ice-cold buffer (0.25 M sucrose, 5 mM Tris/HCl, 1 mM MgCl2; pH 7.4) aerated with 100% O2. Atrium, aorta, and other extraneous tissues were removed. Two hearts were homogenized with five volumes of ice-cold buffer, transferred into a 15 ml conical tube, and centrifuged at 600 g for 10 minutes at 4℃. The supernatant was divided into six to eight 1.5 ml microcentrifuge tubes, and centrifuged at 15,000 g for 10 minutes at 4℃. The supernatant was centrifuged again at 100,000 g for 60 minutes at 4℃. The membrane pellet was dissolved in 1 ml of ice-cold incubation buffer (mM: 50 Tris/HCl, 10 MgCl2; pH 7.5) and used for quantitative analysis. Quantitative analysis was performed with Biochronic Acid Assay (BCA) kit (Pierce, Rockford, IL, USA). Protein concentration was measured by the method of Lowry [13], using a bovine serum albumin (0, 0.25, 0.5, 0.75, 1.0 mg/ml). About 3-4 mg/ml of membranous protein was achieved and kept at -20℃ after freezing with liquid nitrogen.
Each of 0.1, 1, 10, and 100 µM propofol, l-isoproterenol (0.1 µM) or forskolin (3 µM) or GTPγS [guanosine 5"-O-(3-thiotriphosphate)] (1 µM) and the membranous protein (250 µg) were added to cAMP production buffer (mM: 2.5 Na2ATP, 5 MgCl2, 1 Tris-EGTA, 20 creatine phosphate, 50 U/ml creatine phosphokinase, 0.8 IBMX, 50 Tris/HCl, pH 7.5) in a total volume of 500 µl. In the preliminary experiments, we determined the concentrations of these three stimulants which showed maximum tissue levels of cAMP.
To exclude the effect of DMSO on cAMP levels, the concentrations of DMSO used for forskolin were tested. After 10-min incubation at 37℃, the reaction was stopped with 100℃ heat, and the sample was centrifuged at 6,000 g for 20 minutes at 4℃. The cAMP concentration in the supernatant was measured using a cAMP assay kit [cAMP Biotrak Enzymeimmunoassay (EIA) System, Amersham, UK].
Working standard was prepared using the standard for acetylation assay, anti-serum, cAMP peroxidase conjugate and wash buffer provided by cAMP assay kit. Samples were diluted 100 times in assay buffer [0.05 M sodium acetate (pH 5.8), 0.02% bovine serum albumin, 0.01% preservative]. Acetylation reagent mixed with two volumes of triethylamine and one volume of acetic anhydride was added to all standards (0-128 fmol) and samples. One hundred µl of cAMP antiserum was added to 96 well plates coated with secondary antibody (except blank and non-specific binding wells), and 50 µl of samples or acetylation standard were added to each well. Assay buffer alone (150 µl) was added to non-specific binding wells. Plates were incubated for 60 minutes at 4℃. cAMP peroxidase conjugate (100 µl) was added to all wells except blanks and plates were incubated for 60 minutes at 4℃. Wells were aspirated and washed 4 times with 400 µl wash buffer and TMB substrate (150 µl) was added. After 1-hour shaking at room temperature, the reaction was stopped with 1 M sulfuric acid (100 µl). cAMP concentration was measured at 450 nm with a spectrophotometer (ELISA Reader, Versamax, Molecular Devices, Union City, CA, USA). The experiment was performed in duplicate.
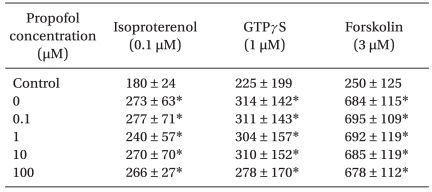
Whereas tissue cAMP levels produced by isoproterenol (0.1 µM), GTPγS (1 µM), and forskolin (3 µM) in guinea pig myocardium were increased when compared to baseline values before treatment of each stimulant, application of propofol (0.1, 1, 10, and 100 µM) did not alter the tissue cAMP levels when stimulated by isoproterenol (n = 10), GTPγS (n = 6), and forskolin (n = 6) (Table 1) (NS).
DMSO (3 µM) did not produce any change in the tissue cAMP levels (n = 8, NS) (control: 489 ± 183 fmol/mg protein).
This study shows that either clinically relevant or supraclinical concentrations of propofol have no effect on the tissue cAMP levels evoked by β-adrenoceptor stimulation (l-isoproterenol), G-protein stimulation (GTPγS), and adenylyl cyclase stimulation (forskolin).
In vitro studies using normal cardiac tissue have reported that propofol has negative inotropic effect in guinea pig [1-3] and ferret [4] ventricular myocardium as well as in human atrial muscles [5] at supraclinical concentrations. However, at clinically relevant concentrations, most in vitro studies indicate that propofol exerts little negative inotropic effect [1,9,10]. At supraclinical concentrations, myocardial depression by propofol has been attributed to decreased ICa via sarcolemma [6-8] and decreased Ca2+ uptake by the sarcoplasmic reticulum [9-11]. However, clinically relevant doses of propofol (0.1-10 µM) had no significant effect on steady state ICa [14]. Clinical concentrations after induction with propofol range from 2 µg/ml (11.2 µM) to 15 µg/ml (84 µM) [15]. Considering that protein binding of propofol exceeds 95%, free fractions of propofol are less than 1 µg/ml (5.6 µM).
Zhou et al. [12] in their study using rat ventricular myocardium demonstrated that relatively high concentrations of propofol (25-200 µM) antagonized β-adrenoceptor binding. Although these results suggest direct inhibitory effects of propofol on β-adrenoceptor signal transduction, the mechanism at the cellular level has not been well elucidated. In rat cardiomyocytes, Kurokawa et al. [14] demonstrated that clinically relevant concentrations of propofol (0.1-10 µM) had no significant effect on steady state ICa but attenuated the increased ICa induced by isoproterenol. In their cAMP experiments, whereas propofol attenuated the isoproterenol-induced increase in cAMP production, propofol did not alter the increase in cAMP induced by direct activation of adenylyl cyclase with forskolin. Therefore, they proposed that the inhibitory site of action of propofol is upstream of adenylyl cyclase. These results indicate that propofol-induced depression of the isoproterenol-stimulated increase in [Ca2+]i and shortening of cardiomyocytes are mediated by a decrease in ICa, which suggest that propofol interferes with the β-adrenergic pathway. In contrast, in guinea pig ventricular tissues, we did not observe any attenuation of increased tissue cAMP levels induced by isoproterenol at either clinically relevant or supraclinical concentrations of propofol. The inhibitory potency of propofol in myocardial contractility at supraclinical concentrations has been reported in guinea pig ventricular papillary muscles [1,3,4]. In contrast, propofol, at either clinically relevant or supraclinical concentrations, was devoid of substantial negative inotropic action in rat papillary muscle [1,9]. Whereas Azuma et al. [1], in their study using rat and guinea pig ventricular myocardium, observed that supraclinical concentrations of propofol (600 µM) caused modest shortening of action potential duration, significant shortening was shown in guinea pig preparations, which suggests species difference in sensitivity of ICa to propofol. Considering these different results, species difference may account for the discrepancies observed in the tissue levels of cAMP.
In conclusion, considering that propofol did not alter the tissue cAMP levels when stimulated by isoproterenol, GTPγS, and forskolin, propofol appears to have no effect on the β-adrenoceptor signaling pathway in guinea pig ventricular myocardium.
References
1. Azuma M, Matsumura C, Kemmotsu O. Inotropic and electrophysiologic effects of propofol and thiamylal in isolated papillary muscles of guinea pig and the rat. Anesth Analg. 1993; 77:557–563. PMID: 8368556.
2. Park WK, Lynch C III. Propofol and thiopental depression of myocardial contractility. Anesth Analg. 1992; 74:395–405. PMID: 1539821.
3. Stowe DF, Bosnjak ZJ, Kampine JP. Comparison of etomidate, ketamine, midazolam, propofol, and thiopental on function and metabolism of isolated hearts. Anesth Analg. 1992; 74:547–558. PMID: 1554122.

4. Cook DJ, Housmans PR. Mechanism of the negative inotropic effect of propofol in isolated ferret ventricular myocardium. Anesthesiology. 1994; 80:859–871. PMID: 8024141.

5. Gelissen HP, Epema AH, Henning RH, Krijnen HJ, Hennis PJ, den Hertog A. Inotropic effects of propofol, thiopental, midazolam, etomidate, and ketamine on isolated human atrial muscle. Anesthesiology. 1996; 84:397–403. PMID: 8602672.

6. Buljubasic N, Marijic J, Berczi V, Supan DF, Kampine JP, Bosnjak ZJ. Differential effects of etomidate, propofol, and midazolam on calcium and potassium channel currents in canine myocardial cells. Anesthesiology. 1996; 85:1092–1099. PMID: 8916827.

7. Zhou W, Fontenot HJ, Liu S, Kennedy RH. Modulation of cardiac calcium channels by propofol. Anesthesiology. 1997; 86:670–675. PMID: 9066334.

8. Yang CY, Wong CS, Yu CC, Luk HN, Lin CI. Propofol inhibits cardiac L-type calcium currents in guinea pig ventricular myocytes. Anesthesiology. 1996; 84:626–635. PMID: 8659791.
9. Riou B, Besse S, Lecarpentier Y, Viars P. In vitro effects of propofol on rat myocardium. Anesthesiology. 1992; 76:609–616. PMID: 1550286.

10. Kanaya N, Murray PA, Damron DS. Propofol and ketamine only inhibit intracellular Ca2+ transients and contraction in rat ventricular myocytes at supraclinical concentrations. Anesthesiology. 1998; 88:781–791. PMID: 9523824.
11. Guenoun T, Montagne O, Laplace M, Crozatier B. Propofol-induced modifications of cardiomyocyte calcium transient and sarcoplasmic reticulum function in rats. Anesthesiology. 2000; 92:542–549. PMID: 10691243.

12. Zhou W, Fontenot HJ, Wang SN, Kennedy RH. Propofol-induced alterations in myocardial β-adrenoceptor binding and responsiveness. Anesth Analg. 1999; 89:604–608. PMID: 10475288.

13. Lowry OH, Rosenbrough NJ, Farr AL, Randall RJ. Protein measurement with Folin phenol reagent. J Biol Chem. 1951; 193:265–275. PMID: 14907713.
14. Kurokawa H, Murray PA, Damron DS. Propofol attenuates β-adrenoreceptor-mediated signal transduction via a protein kinase c-dependent pathway in cardiomyocytes. Anesthesiology. 2002; 96:688–698. PMID: 11873046.

15. Shafer A, Doze VA, Shafer SL, White PF. Pharamcokinetics and pharmacodynamics of propofol infusions during general anesthesia. Anesthesiology. 1988; 69:348–356. PMID: 3261954.




 PDF
PDF Citation
Citation Print
Print


 XML Download
XML Download