This article has been
cited by other articles in ScienceCentral.
Abstract
Background
Rocuronium-induced withdrawal movements can be harmful to patients during the induction period. Remifentanil has been reported to reduce these movements effectively. In this study, we determined the EC50 of remifentanil for the prevention of rocuronium induced withdrawal movements in male, female, old and child group.
Methods
We included patients scheduled for general anesthesia and assigned them into 4 groups depending on their age and gender: male group (20-60 yr), female group (20-60 yr), old group (>65 yr) and child group (6-12 yr). Remifentanil was administered by target controlled infusion. Propofol 2 mg/kg was then administered after equilibration between the effect and plasma concentration of remifentanil was reached. After loss of consciousness, rocuronium 0.6 mg/kg was administered. Patient's response to the rocuronium was graded using a 4 point scale in a blinded manner. The EC50 of remifentanil for preventing rocuronium induced withdrawal movements was determined using Dixon's up-and -down method.
Results
The EC50 of remifentanil for preventing rocuronium induced withdrawal movements was 1.8 ± 0.5 ng/ml [95% confidence interval 1.3-2.2] in the male group, 2.3 ± 1.0 ng/ml [1.3-3.2] in the female group, 0.5 ± 0.4 ng/ml [0.2-0.8] in the old group and 2.8 ± 0.8 ng/ml [2.1-3.5] in the child group.
Conclusions
The EC50 of remifentanil for preventing rocuronium induced withdrawal movements was lowest in the elderly and higher in children than male adult patients. No difference in the EC50 of remifentanil was seen between male and female adult patients.
Go to :

Keywords: Remifentanil, Rocuronium, Withdrawal movements
Introduction
Pain from rocuronium injection has been reported to occur in 50-100% of patients [
1-
3]. The pain has been described as intense and burning sensation in conscious patients [
2,
4]. This injection pain can elicit reflex withdrawal movements of the injected hand and arm or generalized movements of the body in unconscious patients [
5]. These withdrawal movements may dislodge or displace intravenous catheter and can be harmful to children during the induction period.
Remifentanil is a synthetic and esterase metabolized opioid. It has a rapid onset and an ultra-short duration of action with a stable, short context-sensitive half time [
6]. It was reported to effectively reduce rocuronium injection pain and withdrawal movements [
7].
The incidence and severity of rocuronium induced pain differ with age and gender. It is estimated to be higher in female [
8,
9] and pediatric patients [
10].
The aim of the present study was to determine and compare the EC50 of remifentatnil to prevent withdrawal movements in male, female, child and old patients.
Go to :

Materials and Methods
After obtaining approval from the institutional review board and written informed consent from the patients or parents, we enrolled 102 ASA physical status I or II patients undergoing general anesthesia. Patients with a history of neurological deficits, allergies to opioids and local anesthetics, or asthma, and those who had received analgesics or sedatives within the previous 24 hr were excluded from the study.
Patients were allocated into 4 groups according to their age and gender: male group (20-60 yr, n = 23), female group (20-60 yr, n = 24), old group (>65 yr, n = 26). child group (6-12 yr, n = 29). All patients were not premedicated. A 20 gauge intravenous (IV) cannula for adults or a 22 gauge IV cannula for children was inserted into the dorsum of the hand without subcutaneous lidocaine infiltration at about 2 h before the induction of anesthesia on the ward. Ringer's lactate solution was chosen for IV fluid for adults and dextrose-saline solution for children. On arrival in the operating room, each patient's electrocardiogram, pulse oximetry and non-invasive blood pressure were monitored and a mask delivering 5 L/min of O
2 was loosely applied on the patients. Atropine 0.01 mg/kg was administrated for preventing bradycardia in the child group. Remifentanil was administrated by target controlled infusion via a syringe pump (Pilot Anesthesia 2, Fresenius vial, France) driven by STELPUMP (Ver.1.07). Minto et al.'s pharmacokinetic model was used [
11]. Preliminary studies suggested an initial target effect site remifentanil concentration of 4.0 ng/ml. After equilibration of plasma and effect site remifentanil concentration, propofol 2 mg/kg was administered. Immediately after loss of consciousness and eyelash reflex, mask ventilation was started with 5 L/min of O
2 and 1% rocuronium at 0.6 mg/kg was administered over 5 s into a port connected directly to the IV cannula. The withdrawal movements were observed by the same blinded investigator using the 4-grading system which has been utilized in several previous studies (
Table 1) [
12]. A grade of 2 or more was regarded as significant movements. After estimating the withdrawal movements, anesthesia was induced with sevofluane 3-5 vol% and O
2 100% and tracheal intubation was performed 120 s later. Anesthesia was maintained with sevoflurane 1-5 vol% and 50% nitrous oxide in oxygen.
Table 1
Grading of Withdrawal Movements


The EC
50 of remifentanil for preventing rocuronium induced withdrawal movements was determined by a modification of Dixon's up-and-down method [
13]. If the patient's response was grade 2 or 3, the target concentration of remifentanil in the next patient was increased by a step of 0.5 ng/ml. If the patient's response was grade 0 or 1, a decrease of 0.5 ng/ml was made. The process was repeated until the seventh cross-over point (grade 2,3/0,1) was obtained. The midpoint was defined as the mean cross-over concentration. The EC
50 was defined as the mean cross-over mid point in each group.
Side effects such as bradycardia, hypotension, O2 desaturation and difficulty in mask ventilation due to chest rigidity were recorded. Bradycardia and hypotension were defined as a decrease of >20% from their respective preoperative value. O2 desaturation was defined as SpO2 decreasing to <90%.
Statistical analyses were performed using SPSS 11.0. Probit analysis was used for calculating the confidence interval and plotting the dose response curve. Values are expressed as mean ± SD or number of patients. P value of <0.05 was considered to be statistically significant.
Go to :

Results
Table 2 presents the patients' demographic data.
Table 3 presents the hemodynamic data during the induction period. There were no cases of significant hypotension, bradycardia or O
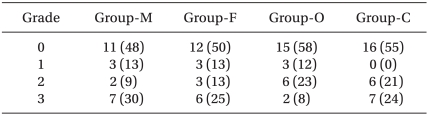
2 desaturation. The incidence of significant withdrawal movements was 39% (9/23) for the male group, 38% (9/24) for the female group, 31% (8/26) for the old group and 45% (13/29) for the child group in all the study remifentanil concentration.
Table 4 presents the grade of withdrawal movements in each group.
Table 2
Characteristics of Patients
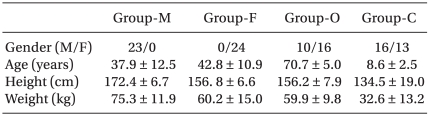

Table 3
Mean Blood Pressure and Heart Rate during Study Period
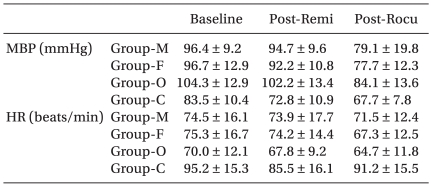

Table 4
Incidence and Grade of Withdrawal Movements induced by Rocuroniun Injection

EC50 of remifentanil for preventing rocuronium induced withdrawal movements was 1.8 ± 0.5 ng/ml [95% confidence interval, 1.3-2.2] in the male group, 2.3 ± 1.0 ng/ml [1.3-3.2] in the female group, 0.5 ± 0.4 ng/ml [0.2-0.8] in the old group and 2.8 ± 0.8 ng/ml [2.1-3.5] in the child group.
The old group required significantly lower effect site concentration of remifentanil for preventing rocuronium withdrawal movements than the other groups (vs. child group P = 0.00, vs. male group P = 0.01, vs. female group P = 0.00). The child group required significantly higher effect site concentration than the male group (P = 0.04). There was no significant difference in the effect site concentration of remifentanil between the male group and female group (P = 0.56) (
Fig. 1,
2).
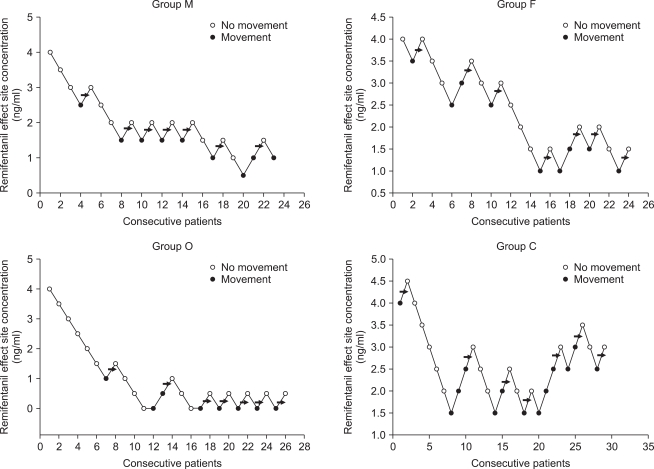 | Fig. 1Consecutive target remifentanil concentrations for determining the EC50. The arrow represents the mean remifentanil concentration when crossing from significant movements (grade 2,3) to no movements (grade 0,1). The average of these concentrations is the EC50. EC50 is 1.8 ± 0.5 ng/ml in male adult, 2.3 ± 1.0 ng/ml in female adult, 0.5 ± 0.4 ng/ml in old and 2.8 ± 0.8 ng/ml in child group. 
|
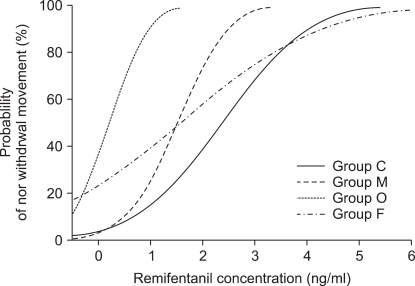 | Fig. 2Dose response curves plotted from probit analysis of individual remifentanil concentration and respective reaction to rocuronium injection. M: male, F: female, O: old, C: child. 
|
Go to :

Discussion
The mechanism of rocuronium induced pain remains unclear. It has been suggested that aminosteroidal neuromuscular blocking drugs such as rocuronium and vecuronium, induce pain by directly activating the C-nociceptor [
14]. Because rocuronium is less potent neuromuscular blocking drug than vecuronium, a higher dose or concentration is required to induce muscle relaxation. This higher dose or concentration has been suggested as the reason for more frequent and severe pain with rocuronium injection [
14].
Pretreatment with opioid has been known to effectively prevent rocuronium induced withdrawal movements [
6,
11,
13]. The central and peripheral sites of action of opioids participate in reducing injection pain but the main site of action is central [
15,
16]. Therefore, the 90 s time interval between the injection of remifentanil and rocuronium was recommended to allow for the opioid's maximal effect.
Previous studies suggested that the withdrawal movements in children are more intense than in adults [
10]. Our study showed that children required a higher remifentanil concentration for preventing withdrawal movements than in the male adult group and old group. Because the rocuronium induced withdrawal movements can be of harm to children during the induction period, appropriate pretreatment is required. An opioid, especially remifentanil, is more appropriate than other drugs in preventing withdrawal movements in pediatric patients [
7]. The absence of side effects such as bradycardia, hypotension and O
2 desaturation, as was noted in this study, makes remifentanil a better choice in preventing withdrawal movements.
The old group required a lower remifentanil concentration than the other groups in our study. This can be explained in part by the pharmacokinetic and pharmacodynamic changes of remifentanil among the elderly. Age is inversely related to the central volume of distribution, clearance, and potency of remifentanil [
17].
Female patients complained of more severe pain than male [
9,
18]. Kim et al. [
10] reported that females show rocuronium induced withdrawal movements 2.1 times more frequently than males in the adult group but there is no gender influence in children. It has been suggested that the gender difference in severity and incidence of rocuronium induced pain in adults may be related with sex hormones. We however, did not find any difference in the remifentanil EC
50 between male and female adult groups. The opioid is known to be more potent in female than male [
19]. But Minto et al reported that the potency of remifentanil was not influenced by gender [
11].
In conclusion, we found that the EC50 of remifentanil for preventing rocuronium induced withdrawal movements is lowest in the elderly in the all groups and higher in children when compared to adult male patients. No such difference was seen between male and female adult patients.
Go to :






 PDF
PDF Citation
Citation Print
Print







 XML Download
XML Download