Introduction
Materials and Methods
1. Acquisition of primary cells and the culture and passage of PDX-OS cells
1) Acquisition of human OS tissue
2) Primary PDX-OS cell acquisition, culture, and passage
Three pieces of OS tissue approximately 1 cm3 in size were obtained from OS patients during surgery and were immediately placed in a sterile container filled with ice-cold sterile saline and maintained on ice. After cleaning, the samples were placed in a 15-mL sterile centrifuge tube filled with 6 mL medium and transferred on ice to the laboratory for further preparation.
The OS tissue blocks were cut into pieces 2 mm3 in volume with sterile shears and then cleaned with sterile phosphate buffered saline (PBS) before they were trimmed into fragments with sterile shears and then rinsed with PBS until the liquid was clear and free of turbidity and oil droplets.
OS tissue samples were transferred into 3 mL serum-free culture medium containing 1 mg/mL collagen hydrolase and enzymolyzed in a water bath at 37°C for 30 minutes.
After enzymolysis, 3 mL medium containing 10% fetal bovine serum (FBS) was added to neutralize the remaining collagen hydrolase activity. The samples were centrifuged for 5 minutes at room temperature at 350 ×g, and the supernatant was discarded. This centrifugation procedure was repeated twice.
Cell pellets obtained after the final centrifugation were resuspended in PBS, counted and then added to a T25 cell culture flask containing culture medium. The medium was changed every 2 to 3 days or when the medium color changed. Cells that underwent amplified growth were passaged and stored. The PDX-OS1 and PD-OX2 lines were derived from OS tissue with an excellent response to neoadjuvant chemotherapy, and the PDX-OS3 line was derived from CDDP chemotherapy-resistant OS tissue.
All cell experiments were conducted within 10 passages after primary cell harvest. PDX-OS1 and PDX-OS2 cells were cultured in Dulbecco’s modified Eagle’s medium (Invitrogen, Carlsbad, CA) supplemented with 10% FBS and 1% penicillin and streptomycin. The incubator was set at 37°C and 5% CO2. PDX-OS3 cells were cultured under pressure using the same medium with 5 μM CDDP.
3) Conventional human OS cell and UM-SCC cell culture
4) Chemical reagents, drug treatment, and corresponding antibodies
5) Cell transfection
2. Cell toxicity and proliferation assay
3. Western blot
4. Coimmunoprecipitation assay
5. Confocal immunofluorescence assay
6. Cell cycle assay
7. Animal experiments
8. Statistical methods
Results
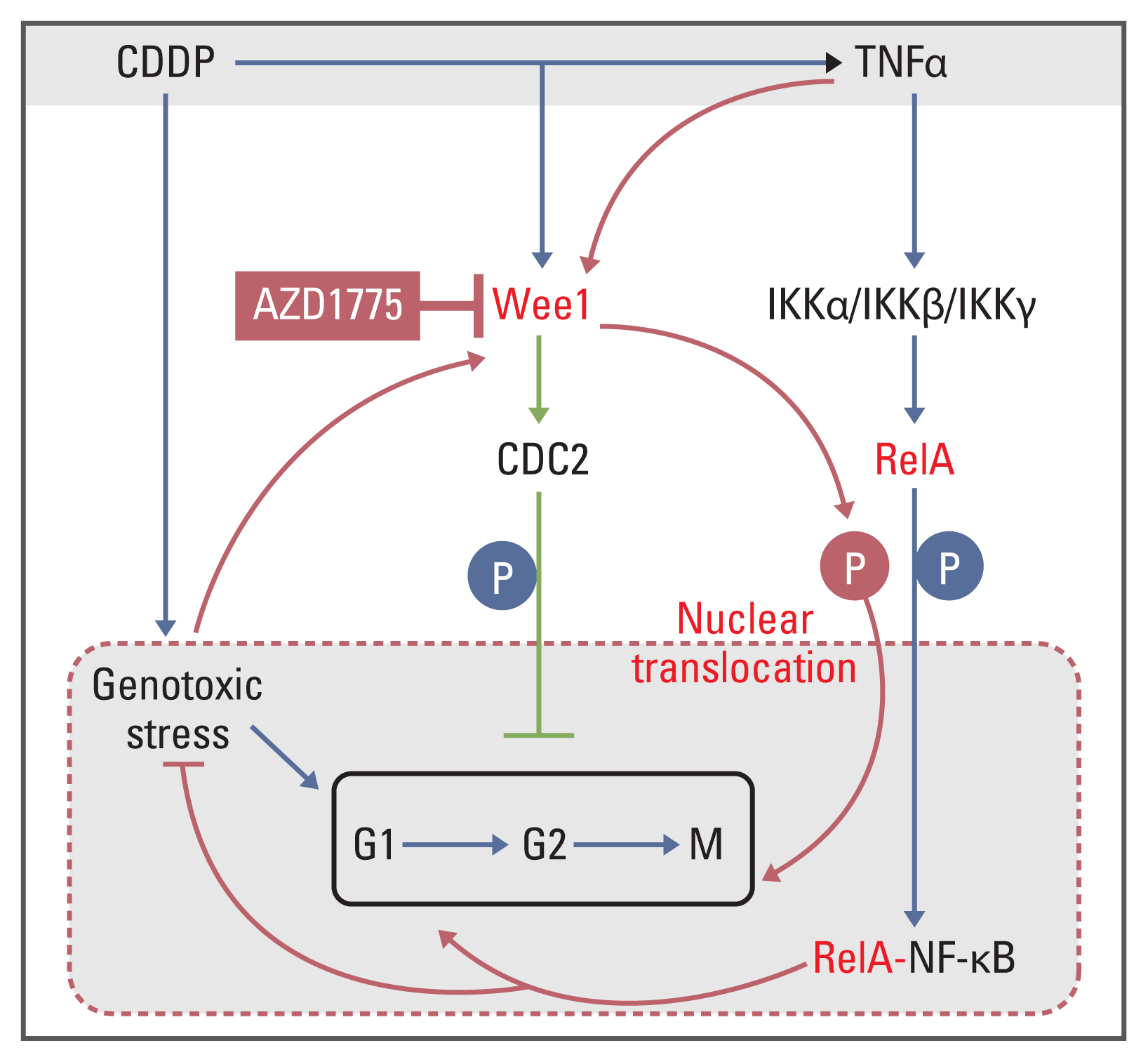
1. Heterogeneity of the expression of Wee1/CDC2 and NF-κB signaling pathway molecules exists and is affected by the antitumor drug CDDP in tumor cells with variable IC50 values of AZD1775 and CDDP
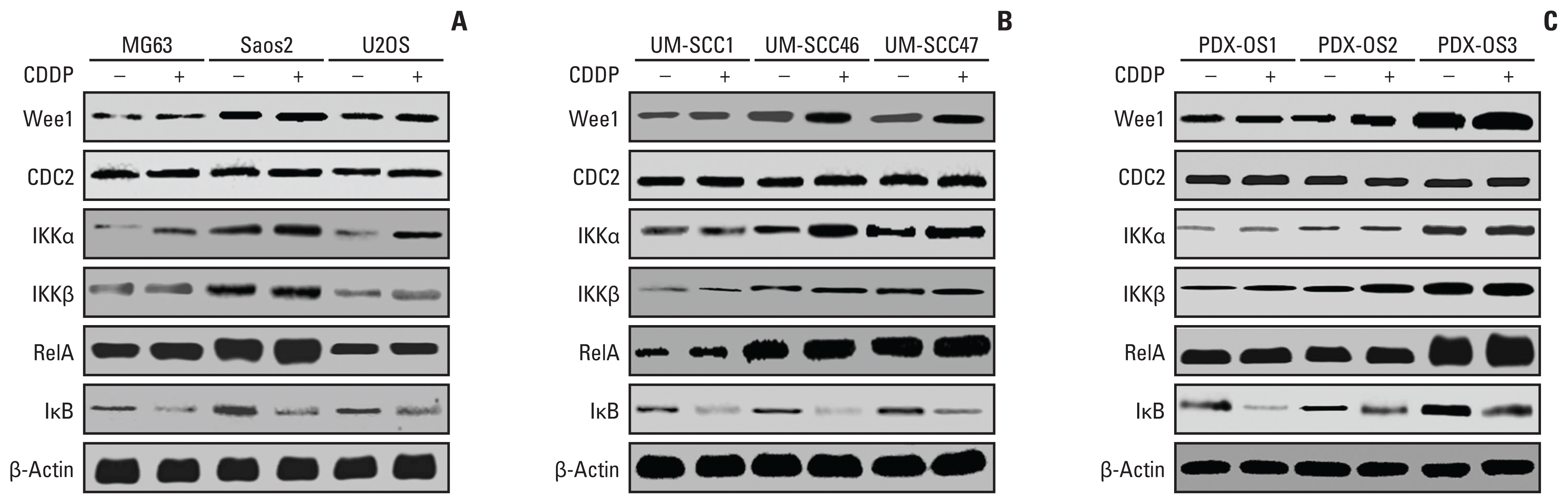 | Fig. 1Wee1/CDC2 and nuclear factor κB (NF-κB) signaling pathway molecules in parental tumor cells had heterogeneous expression levels and were upregulated by cisplatin (CDDP). Tumor cells were seeded in a 10-cm cell culture dish (1×105 cells/dish) and cultured for 48 hours. Cells were treated with CDDP (25 μg/mL) in fresh medium for 6 hours when they reached 70%–80% confluence and then harvested. Parental cells were treated with fresh medium alone. Protein quantification was performed after cell lysis, and 30 μg protein per well was subjected to western blot analysis. The protein expression levels of Wee1/CDC2 and NF-κB pathway molecules IKKα, IKKβ, RelA, and IκB in conventional cells were significantly different, with the hierarchy Saos2 > U2OS > MG63 for OS cell lines (p < 0.01) (A), UN-SCC46 > UM-SCC47 > UM-SCC1 for head and neck squamous cell lines (p < 0.01) (B), and PDX-OS3 > PDX-OS2 > PDX-OS1 for PDX OS cell lines (p < 0.01) (C). CDDP significantly increased the protein expression levels of Wee1, IKKα, IKKβ, and RelA and decreased that of IκB in these tumor cells (p < 0.01). |
Table 1
Cells were seeded into 96-well plates at 5,000 cells per well. Each sample had six replicates, and the data were calculated and are expressed as the mean plus standard deviation (X±SD). Inhibitory rates were calculated by Microsoft Excel, and the half-maximal inhibitory concentration (IC50) values were calculated using PRIM 5.0 software.
2. High basal protein expression of the Wee1/CDC2 axis and NF-κB signaling pathway components was activated under TNFα or CDDP treatment, and this activation was abolished by Wee1 inhibition
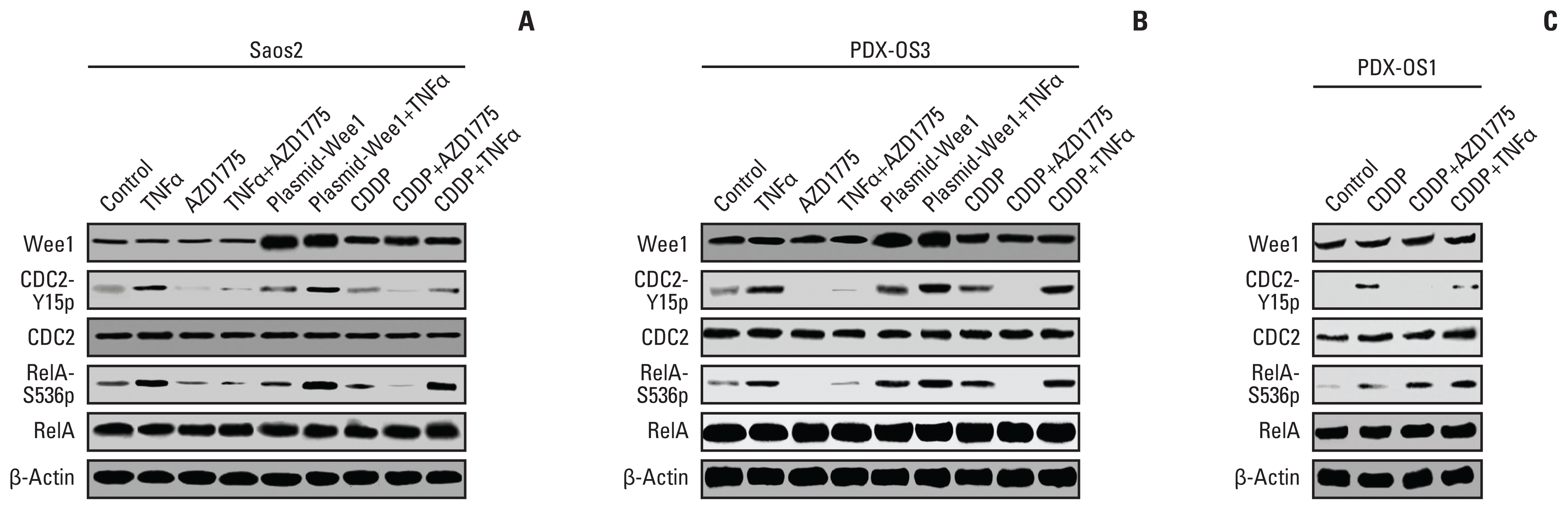 | Fig. 2AZD1775 abolished the effects of tumor necrosis factor α (TNFα), cisplatin (CDDP), and Wee1 treatment in CDDP-tolerant osteosarcoma cells by amplifying the phosphorylation of both CDC2-Y15 and RelA-S536. Cells were seeded at 1×105 in a 10 cm cell culture dish, cultured for 48 hours, and harvested when the cells reached 70%–80% confluency. The cells were transfected with the plasmid Wee1 for 24 hours, after which they were treated with CDDP or TNFα for 6 hours. Vehicle treatment was used as a control. After cell lysis, the protein concentration was quantitated, and western blotting was performed with 25 μg of protein per sample. (A) In Saos2 cells, the levels of phosphorylated CDC2-Y15 (CDC2-Y15p) and RelA-S536 (RelA-S536p) in the TNFα treatment, Plasmid-Wee1 treatment and CDDP treatment groups were significantly amplified compared with that of the control group (p < 0.01) and were significantly reduced in the AZD1775 treatment group (p < 0.01). Compared with the TNFα treatment and Plasmid-Wee1 treatment groups, the Plasmid-Wee1+TNFα treatment group showed significantly increased CDC2-Y15p and RelA-S536p levels (p < 0.05). The levels of CDC2-Y15p and RelA-S536p in the TNFα+AZD1775 treatment group were significantly lower than those in the TNFα treatment group (p < 0.01), and the levels were significantly lower in the CDDP+AZD1775 treatment group than in the CDDP treatment group (p < 0.01). Moreover, these levels were significantly higher in the CDDP+TNFα treatment group than in the CDDP treatment group (p < 0.01). (B) In CDDP-resistant PDX-OS3 cells, the levels of CDC2-Y15 and RelA-S536 in each treatment group were basically consistent with those in the corresponding Saos2 cells. Interestingly, CDC2-Y15p and RelA-S536p levels were more obviously decreased in the treatment groups containing AZD1775, with statistical significance (p < 0.01). (C) Although the levels of CDC2-Y15p and RelA-S536 in PDX-OS1 cells were significantly increased in the CDDP-treated group compared to the control group, the levels of CDC2-Y15p in the CDDP+AZD1775 treatment group were decreased (p < 0.01), which was accompanied by increased RelA-S536 phosphorylation. In addition, the levels of RelA-S536p were amplified in the CDDP+TNFα group (p < 0.01), accompanied by a reduction in CDC2-Y15p levels. This is consistent with the results in U2OS and MG63 cells (data not shown). |
3. Physical binding of Wee1 to RelA in OS cells is modulated by CDDP, and Wee1 modulates the translocation of RelA into the nucleus
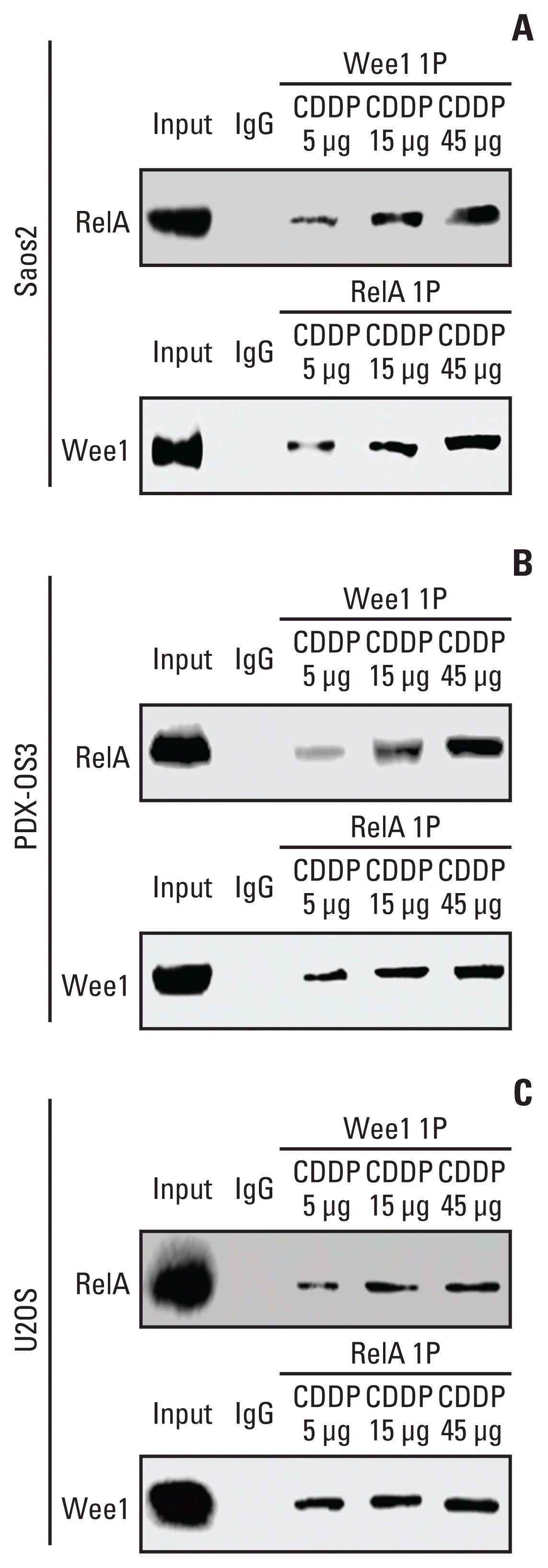 | Fig. 3The interaction between Wee1 and RelA, as indicated by coimmunocipitation assays in osteosarcoma cells. Cells were seeded at 1×105 in a 10 cm cell culture dish and cultured for 48 hours. Cells were treated with different concentrations of cisplatin (CDDP; 5, 15, and 45 μg/mL) for 6 hours when they reached 70%–80% confluence and then harvested. Whole-cell lysate was used as the positive control, and IgG was used as the negative control. Protein quantification was performed after cell lysis, and the corresponding immunoprecipitants were obtained according to the method instructions, with 30 μg of immunoprecipitants per sample subjected to western blotting. In Saos2 cells (A), Wee1 binding to RelA showed a CDDP dose-dependent effect when treated with three different concentrations of CDDP. This result was reproduced in PDX-OS3 (B) and U2OS cells (C). |
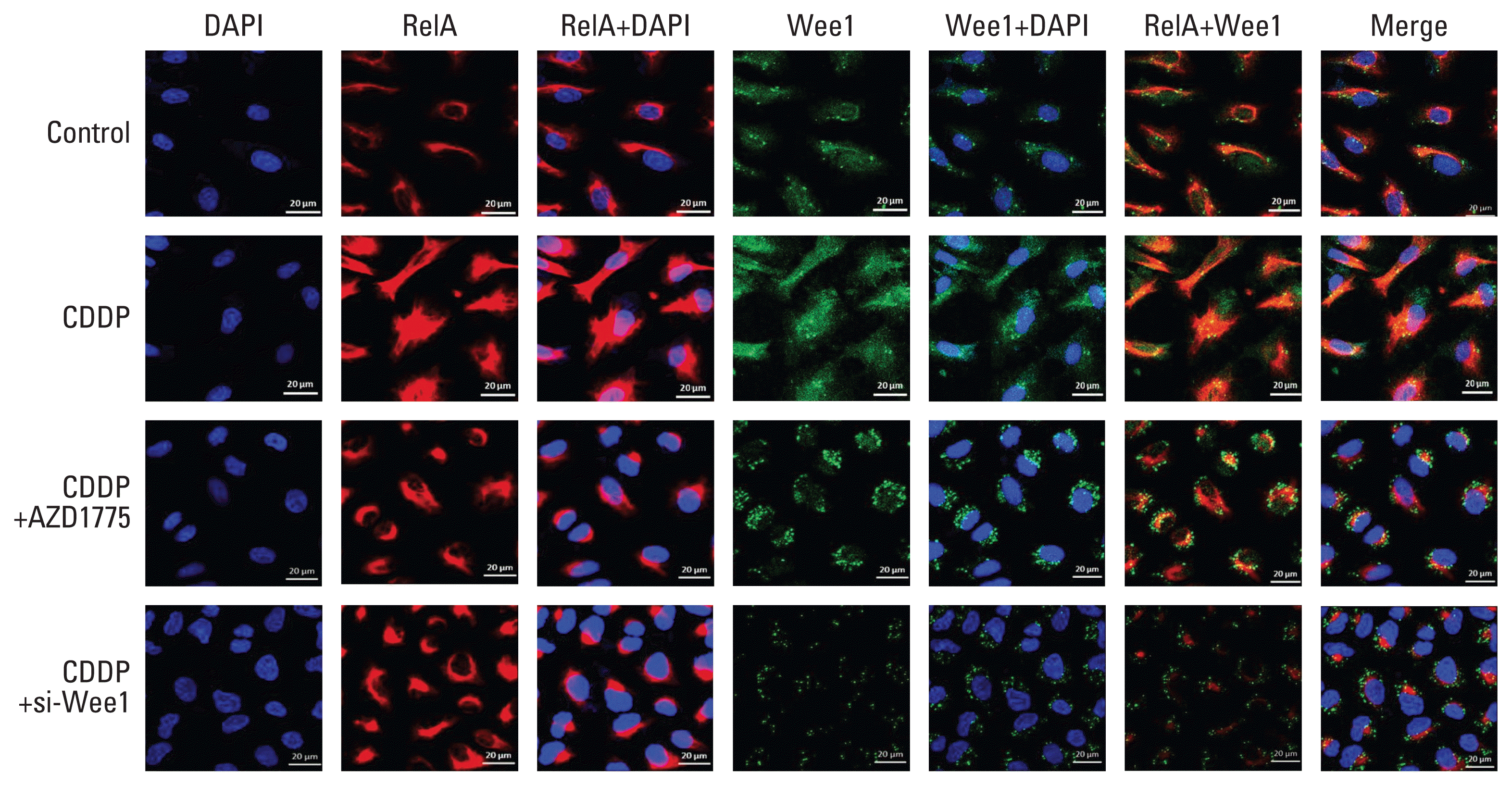 | Fig. 4Wee1 affects the nuclear translocation of RelA in cisplatin (CDDP)-resistant osteosarcoma cells. In confocal immunofluorescence experiments, PDX-OS3 cells were seeded in a cell culture plate at 2×104, cultured for 48 hours, treated with CDDP (25 μg/mL) for 6 hours when the cells reached 50%–60% confluence, and harvested. DMSO was used as a negative control. In PDX-OS3 cells, both Wee1 (green) and RelA (red) fluorescence signals were enhanced in the CDDP treatment group compared to the control group (row 1). Compared with the CDDP treatment group (row 2), the CDDP+AZD1775 group (row 3) showed Wee1 green signal distribution and aggregation mainly in the cytoplasm, while the RelA red signals were congested in the periphery of the nucleus and surrounded by Wee1 green signals. Furthermore, the RelA red signal in the nucleus was reduced. In addition, compared to the CDDP group, the CDDP+si-Wee1 treatment group (row 4) showed that the Wee1 green signal was obviously reduced, while the RelA red signal was also congested around the nucleus and was significantly reduced in the nucleus, which is consistent with the results from the CDDP+AZD1775 group. Scale bars=20 μm. |
4. Inhibition of Wee1 restores the effect of CDDP on cell cycle progression, proliferation and survival in CDDP-resistant OS cells
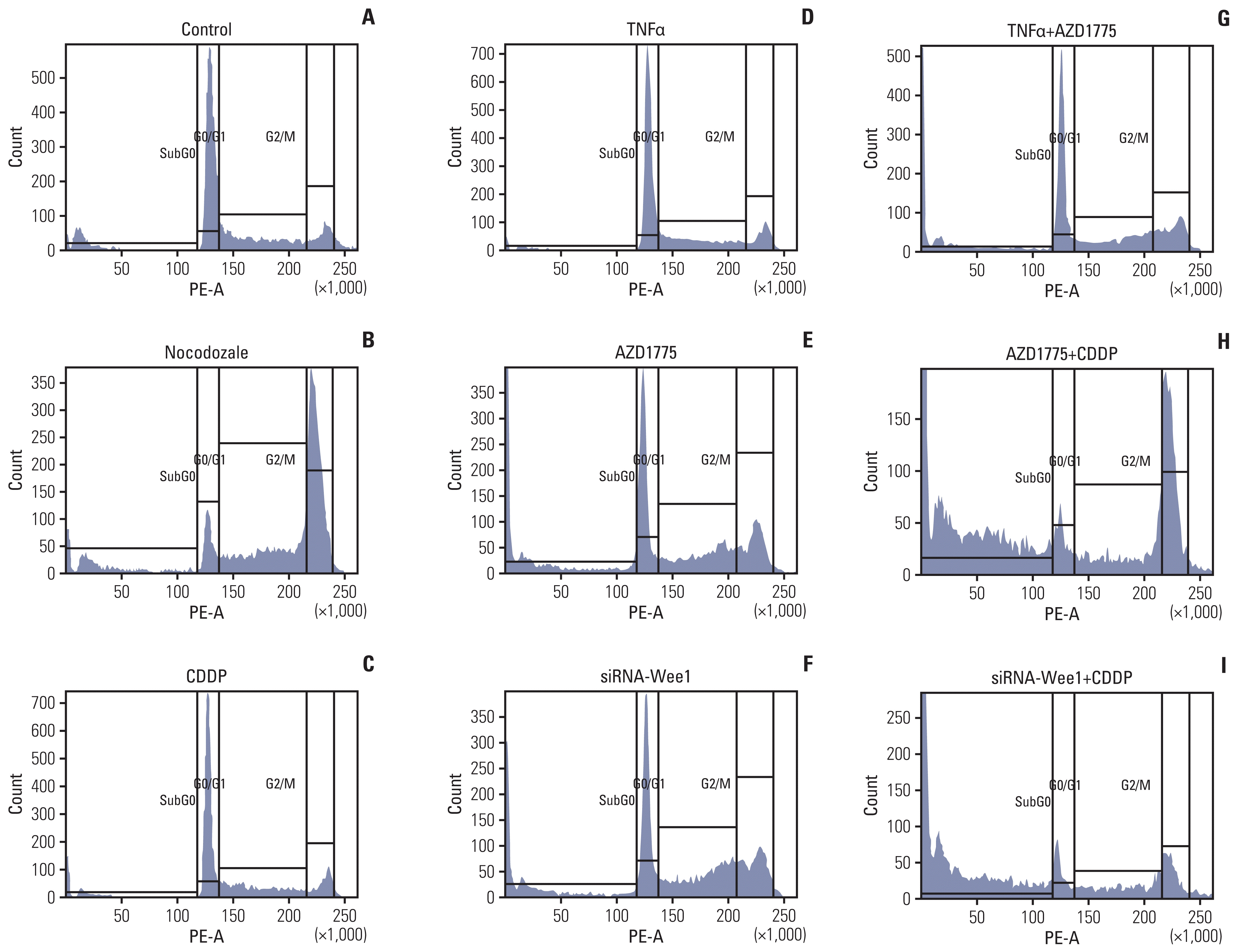 | Fig. 5Inhibition of Wee1 restores the effects of cisplatin (CDDP) on cell cycle progression and survival in Saos2 cells. In the cell cycle assays, Saos2 cells were seeded in a 10 cm cell culture dish at 1×105 and cultured for 48 hours. Cells were treated with si-Wee1 transfection or CDDP, nocodozale, tumor necrosis factor α (TNFα) and other treatments for 24 hours when the cells reached 70%–80% confluence, after which they were harvested. Vehicle treatment was used as a negative control (A), and the cell cycle G2 inhibitor nocodozale was used as a positive control (B). The proportion of G1 phase cells in the nocodozale treatment group was significantly decreased, while the proportion of G2 phase cells was significantly increased (p < 0.01). Compared with the negative control group, there was no significant difference in the proportion of cells in the different phases in the CDDP (C) or TNFα groups (D). Compared with the negative control and positive control groups, the proportion of cells in G2 and sub-G0 phases (cells dying after mitotic catastrophes) and the proportion of cells in G1 phase in the AZD1775 group (E) and siRNA-Wee1 group (F) were significantly increased, whereas the proportion of G1 phase cells was significantly decreased (p < 0.01). Compared with the TNFα treatment group (D), the AZD1775+TNFα treatment group (G) showed an increased proportion of G2 phase cells but a significant decrease in the proportion of sub-G0 phase cells (p < 0.01). Compared with the CDDP treatment group, the AZD1775+CDDP group (H) and siRNA-Wee1+CDDP group (I) showed significant decreases in the proportion of G1 phase cells (p < 0.01) and significant increases in the proportion of sub-G0 cell fragments (p < 0.01). |
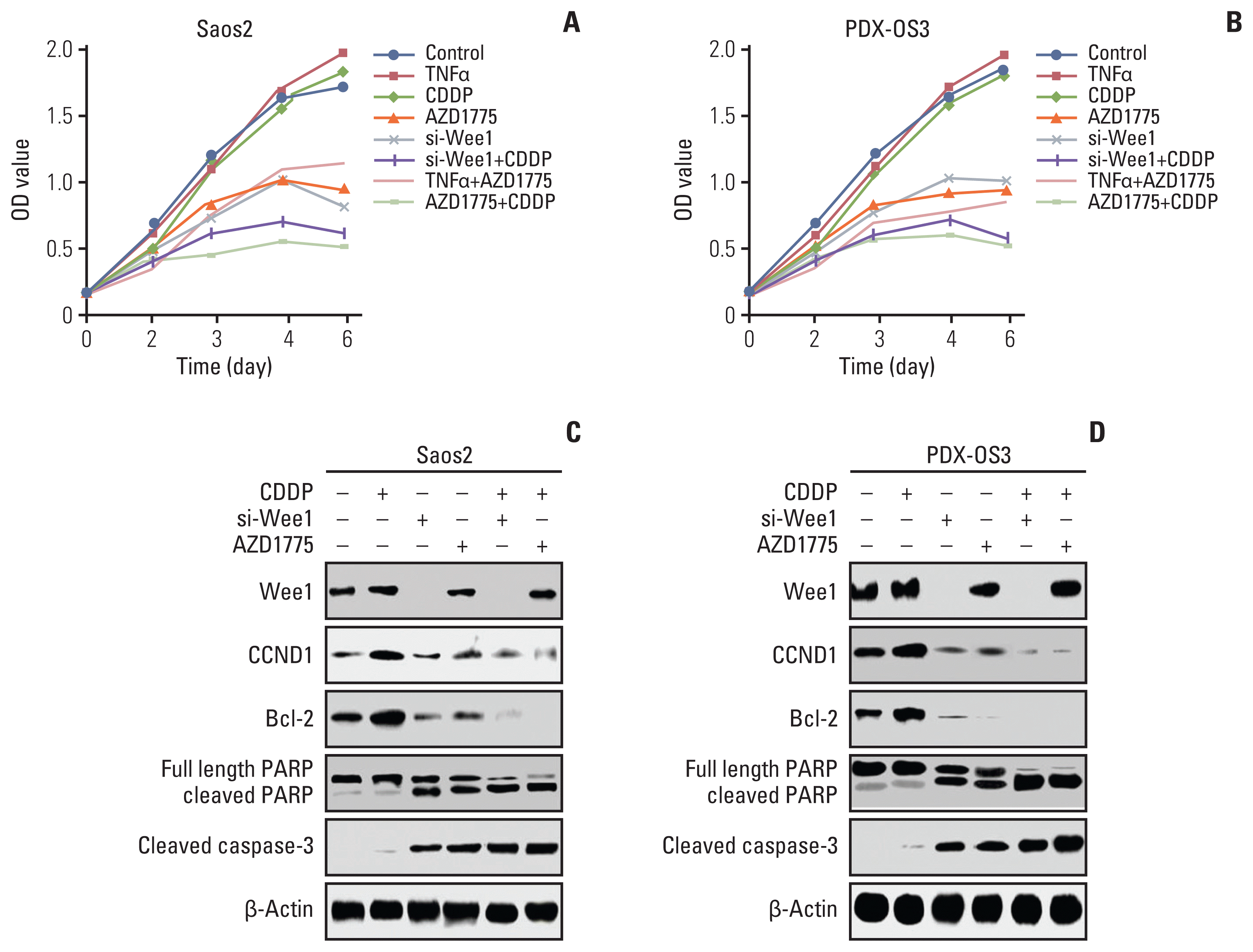 | Fig. 6Inhibition of Wee1 restores the effects of cisplatin (CDDP) on cell proliferation and activation of cell death-related molecular pathways in osteosarcoma cells with high tolerance to CDDP. In the Saos2 cell proliferation experiment (A), there was no significant difference in the cell optical density (OD) value between the tumor necrosis factor α (TNFα) and CDDP treatment groups and the control group. Compared with the control and CDDP treatment groups, the AZD1775 treatment and siRNA-Wee1 treatment groups showed significantly smaller OD values (p < 0.05). Compared with the TNFα group, the AZD1775+TNFα group also showed significantly lower OD values (p < 0.05). Compared with the CDDP treatment group, the CDDP+AZD1775 treatment group and CDDP+si-Wee1 treatment group both had the most significant decreases in OD values (p < 0.01). In the PDX-OS3 cell proliferation experiment (B), similar to those in Saos2 cells, the OD value was significantly lower in the AZD1775 and siRNA-Wee1 treatment groups than in the control group (p < 0.05) and was significantly lower in the AZD1775+TNFα treatment group than in the TNFα treatment group (p < 0.05). Compared with the CDDP treatment group, the CDDP+AZD1775 treatment group and CDDP+si-Wee1 treatment group both had the most significant decreases in the OD value (p < 0.01). In the Saos2 western blot assay (C), compared with the control group, the CDDP treatment group showed a significant increase in the levels of the NF-κB downstream molecules Bcl-2 and CCND1 (p < 0.05), while the differences in the expression of the apoptosis-related proteins cleaved poly(ADP-ribose) polymerase (PARP) and cleaved caspase-3 were not statistically significant. Compared with the control group, the AZD1775 or siRNA-Wee1 treatment group showed significantly lower Bcl-2 and CCND1 expression levels (p < 0.05) and significantly higher cleaved PARP and cleaved caspase-3 levels (p < 0.05). Moreover, Bcl-2 and CCND1 levels were significantly decreased in the CDDP+AZD1775 group and CDDP+si-Wee1 group (p < 0.01), which were accompanied by simultaneous increases in the apoptosis-related proteins cleaved PARP and cleaved caspase-3 (p < 0.01). In PDX-OS3 cells (D), consistent with the results in Saos2 cells, Wee1 inhibition treatment significantly decreased Bcl-2 and CCND1 expression and simultaneously increased cleaved PARP and cleaved caspase-3 levels, especially in the CDDP combined with Wee1 inhibition group. |
5. AZD1775 inhibits the growth of CDDP-resistant PDX-OS, restores the cytotoxicity of CDDP toward PDX-OS, and prolongs the median survival of the host
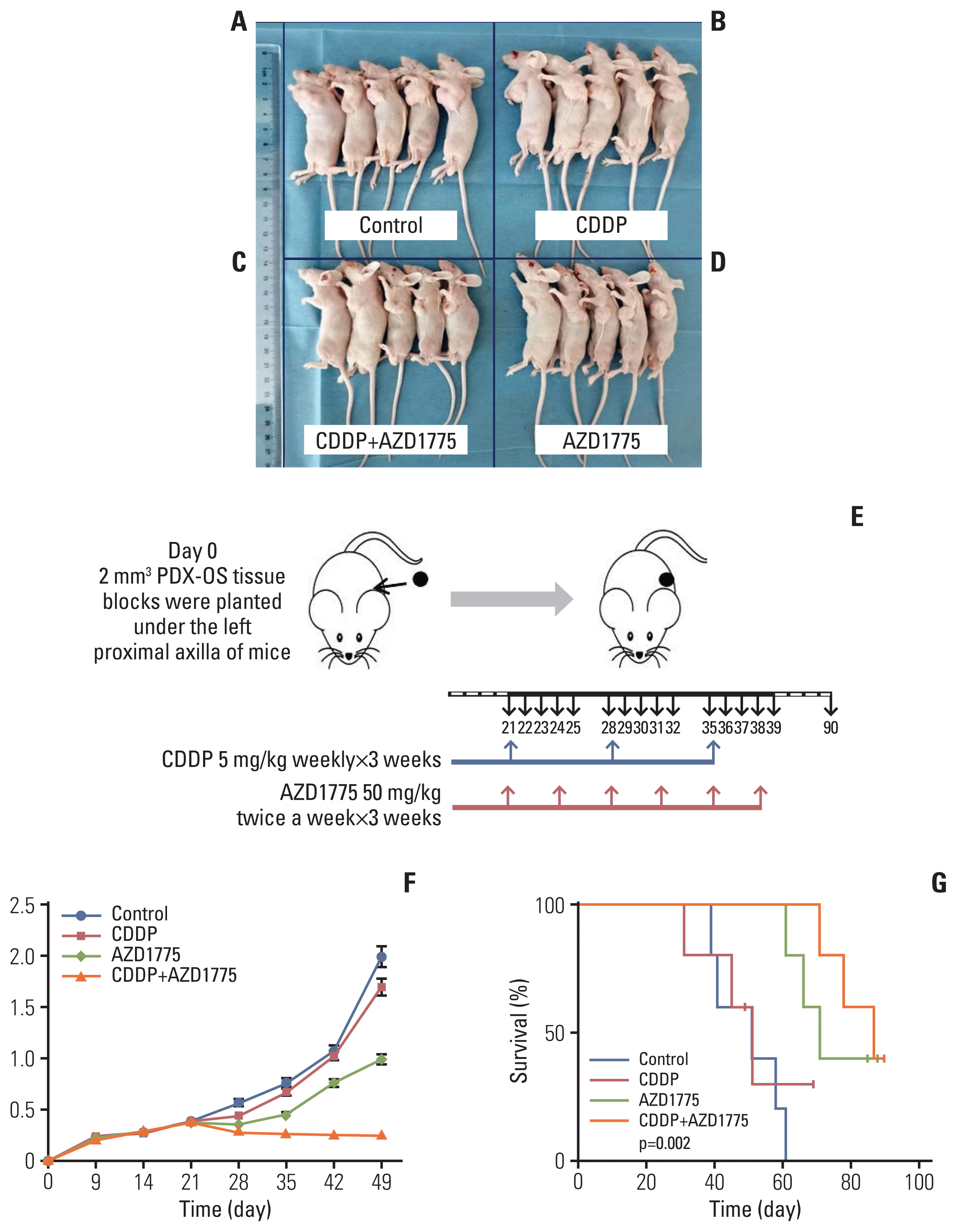 | Fig. 7AZD1775 combined with cisplatin (CDDP) treatment enhanced and restored the therapeutic effect of CDDP, resulting in an extended median survival of the host. PDX-OS3 cells were transplanted into female nude mice and grown for 4 weeks, and the mice were randomly divided into four groups (5 mice per group) (A-D). CDDP 5 mg/kg was intraperitoneally injected once a week, and AZD1775 50 mg/kg was intragastrically administered twice a week for 3 weeks (E). Tumor volume changes were recorded regularly (F), and survival analysis was performed (G). Compared with the control group (A), the CDDP treatment group (B) showed a slightly decreased tumor volume, but the difference was not significant (p > 0.05). However, the tumor volume was significantly decreased in the CDDP+AZD775 group (C) (p < 0.01). The tumor volume was also significantly reduced in the AZD1775 treatment alone group (D) compared with the control group or CDDP treatment group (p < 0.05). The median survival of mice in the control, CDDP treatment, AZD1775 treatment and CDDP+AZD1775 treatment groups was 51, 53, 72, and 89 days, respectively. The median survival of the CDDP+AZD1775 treatment group was the longest, and the difference was significant (p < 0.01). |




 PDF
PDF Citation
Citation Print
Print


 XML Download
XML Download