Introduction
Acute promyelocytic leukemia (APL) is a rare and unique subtype of acute myeloid leukemia (AML) characterized by the presence of the recurrent t(15;17) translocation, and accounts for approximately 5%–10% of childhood AML patients [
1–
5]. APL is classified as AML-M3 (hypergranular promyelocytic leukemia) according to the French-American-British classification system because it presents characteristic cells containing bundles of Auer rods (‘faggots’) in the cytoplasm. However, microgranular variant (M3v) form with irregular nucleus and hypo-granulatioin was also found in about 20% of APL [
5]. Pediatric APL patients are known to have a higher frequency of hyperleukocytosis and the M3v subtype at diagnosis compared with adult APL patients [
1,
3,
5,
6].
The fusion of promyelocytic leukemia (PML) gene on chromosome 15 with retinoic acid receptor-alpha (RARα) gene on chromosome 17 expresses the PML-RARα protein, which blocks cell differentiation and interferes with apoptosis and tumor suppression. On the presence of the PML-RARα fusion gene in leukemic cells, all-trans retinoic acid (ATRA) induces differentiation of promyeloblasts into neutrophil and arsenic trioxide (ATO) acts both promyeloblast differentiation and apoptosis [
1,
3]. With the introduction of ATRA treatment, the survival rate of APL showed significant improvement [
5]. Several studies have reported that the treatment of pediatric APL based on ATRA and anthracycline chemotherapy results in outcomes similar to those of adult APL [
6–
10]. ATO has the advantage of being less toxic compared to standard chemotherapy. Therefore, it is increasingly used in childhood APL as first-line treatment as well as for reinduction therapy after relapse [
2,
3,
11].
Hemorrhagic diathesis at diagnosis or after the initiation of cytotoxic chemotherapy is a unique feature of APL and is known to be due to disseminated intravascular coagulation or hyperfibrinolysis triggered by APL blasts. Early deaths from bleeding in APL are reported in 10%–20% [
3,
5].
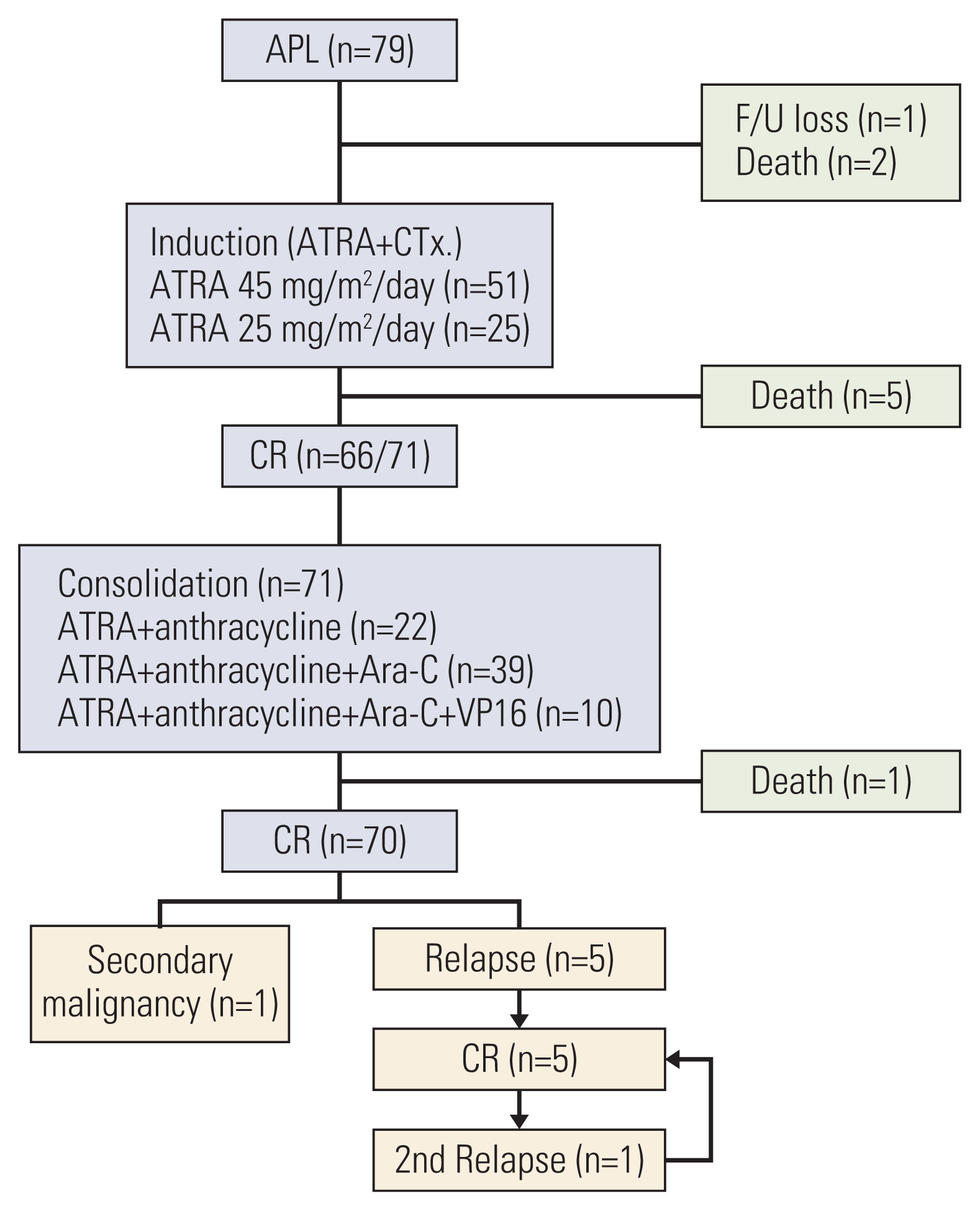
Since APL is a rare disease in children, there have not been many studies on pediatric APL. This study was a nationwide, multicenter, retrospective study with the primary aim of investigating the incidence, clinical characteristics and treatment outcomes of childhood APL in Korea.
Go to :

Discussion
The present retrospective, nationwide, multicenter study evaluated the incidence and survival outcomes of APL in Korean children. The incidence of pediatric APL in Korea was about 10% of
de novo AML patients less than 18 years old in this study. The frequency of APL was similar to results of other studies that have been reported [
6–
9]. The age distribution of patients with APL different from that of other AML patients. While APL is uncommon in the first decade of life, the incidence increases during the second decade reaching a plateau during early adulthood, and diagnosis at age < 1 year is very rare [
3,
10]. The sex ratio of childhood APL varies with study size, and as the study size increased, sex differences became less apparent [
4]. In our study, the median age at diagnosis was 10.6 years (range, 1.3 to 18.0 years), and the male to female ratio was almost the same.
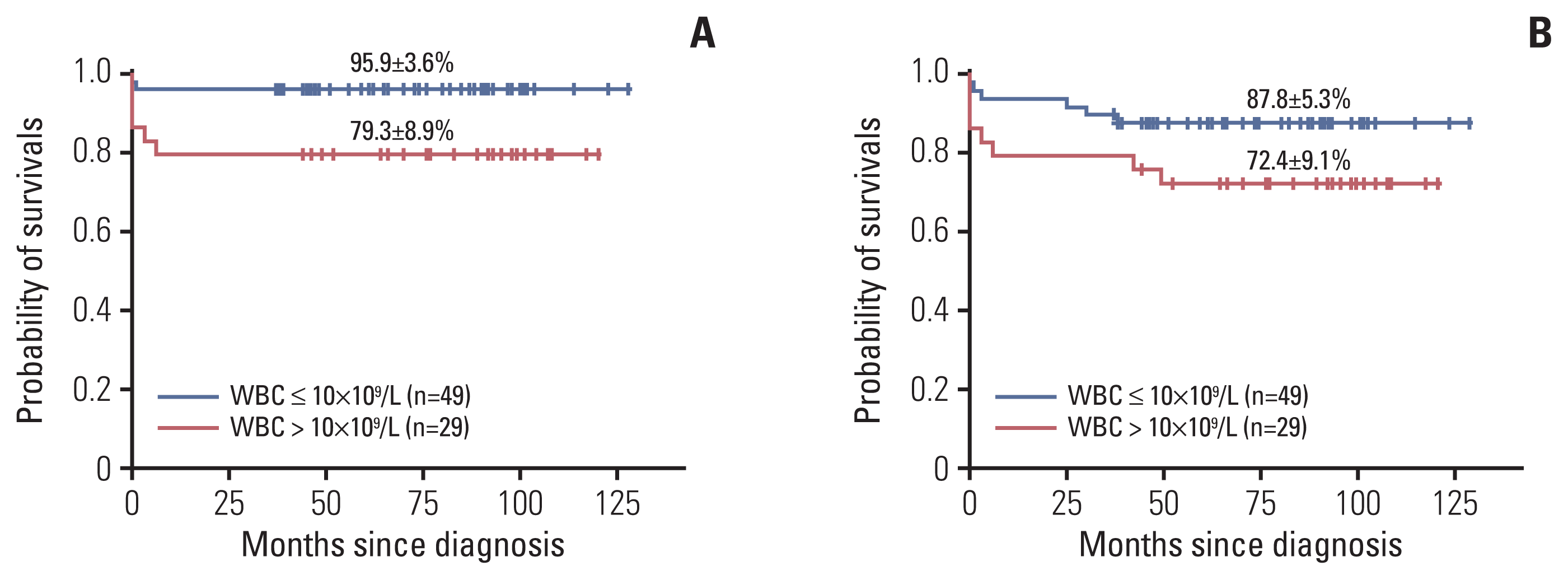
Initial leukocyte count is known as the most important factor in APL, and the patients with an initial WBC count of ≥ 10×10
9/L are classified as a high-risk group for recurrence [
9,
12,
13]. Compared with adult APL patients, pediatric APL patients more frequently present hyperleukocytosis at diagnosis (approximately 40% in children vs. 20%–25% in adults) and are more likely to have the M3v subtype [
3,
10]. Nevertheless, several studies of pediatric APL have reported similar survival rates to those of adult APL [
6–
9]. In our study, 38% of patients were classified as high risk, but M3v was observed in only one patient.
Since simultaneous administration of ATRA and anthracycline-containing chemotherapy was established as frontline treatment for APL, favorable outcomes have been reported in several multicenter studies of pediatric APL [
6–
9]. The GIMEMA-AIEOP group studied 124 pediatric APL patients and reported a CR rate of 96%, and the OS and the EFS of 89% and 76%, respectively [
9]. In the PETHEMA group, 66 children were included and reported a CR rate of 92%, and OS and the disease-free survival (DFS) rates of 87% and 82%, respectively [
7]. In a recent ICC-APL-01 trial, the OS and the EFS of 258 pediatric patients were reported as 94.6% and 79.9%, respectively [
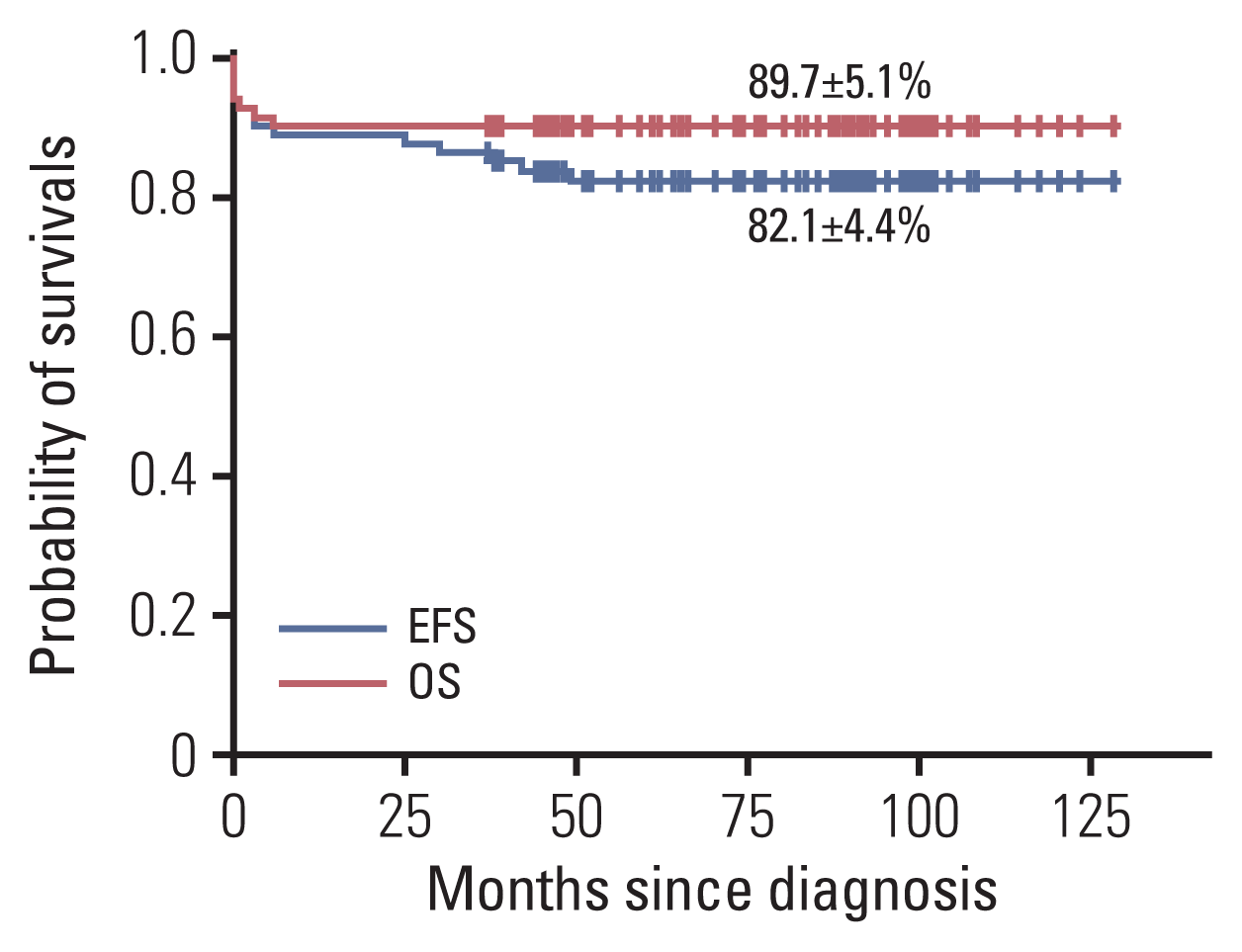
14]. In our study, the survival rates of patients treated with a combination of ATRA and anthracycline-containing chemotherapy were similar to other previously reported results (OS, 89.7±5.1%; EFS, 82.1±4.4%). Although the CR rate of the low-risk group in this study was less than was found in the other risk groups, the final OS and EFS rates were high as the patients who initially achieved PR or NR patients in this group finally achieved CR with reinduction or consolidation chemotherapy.
In the previous study by the PETHEMA group, the patients in the high-risk group had a higher cumulative incidence of relapse (CIR) and a lower DFS than the other groups (CIR 31% vs. 3.5%, p=0.01; DFS 68±24% vs. 96±7%, p=0.01) [
7]. Another study by GIMEMA-AIEOP group also reported that the EFS in the high-risk group was significantly lower than in the other groups (59% vs. 83%, p=0.007) [
9]. In this study, the EFS and the OS in the high-risk group was worse than in the other groups, and the initial high leukocyte count was the only prognostic factor (EFS, 72.4±9.1% vs. 87.8±5.3%, p=0.091; OS, 79.3±8.9% vs. 95.9±3.6%, p=0.020).
One of the major complications of APL is a life-threatening consumptive coagulopathy. This coagulopathy usually develops early in the course of disease before starting APL treatment or within a few weeks, and the CNS and the lungs are the most common areas of fatal bleeding [
2,
5,
15,
16]. In the COG AAML0631 trial, they evaluated 79 pediatric patients, and four early deaths (all high-risk group) and 13 non-fatal but clinically significant coagulopathy events were observed before the end of induction [
15]. Of the 258 patients enrolled in the ICC-APL-01 trial, eight early deaths (1 standard risk and 7 high risk) were observed, and the cause of death was ICH in all eight patients [
14]. In our study, seven (8.3%) early deaths were observed; six patients died from bleeding due to coagulopathy, and one patient died from sepsis. Although one patient died due to cardiogenic shock after the end of induction, almost all deaths occurred prior to treatment or before the end of induction. In addition, four out of six early deaths due to coagulopathy were in the high-risk group, which was a major contributing factor in the significantly reduced OS rate in the high-risk group in this study. Early recognition of disease and early treatment with ATRA will decrease early deaths from disease in APL children.
The side effects of ATRA treatment include RA syndrome, headache, and PTC. RA syndrome is known to be another leading cause of early death in APL, and several studies have shown that the incidence of RA syndrome in pediatric APL is about 10%–20%, similar to that of adult APL [
2,
6–
9]. On the other hand, other side effects related to ATRA, such as headache and PTC, have been reported to occur more frequently in children than in adults [
6,
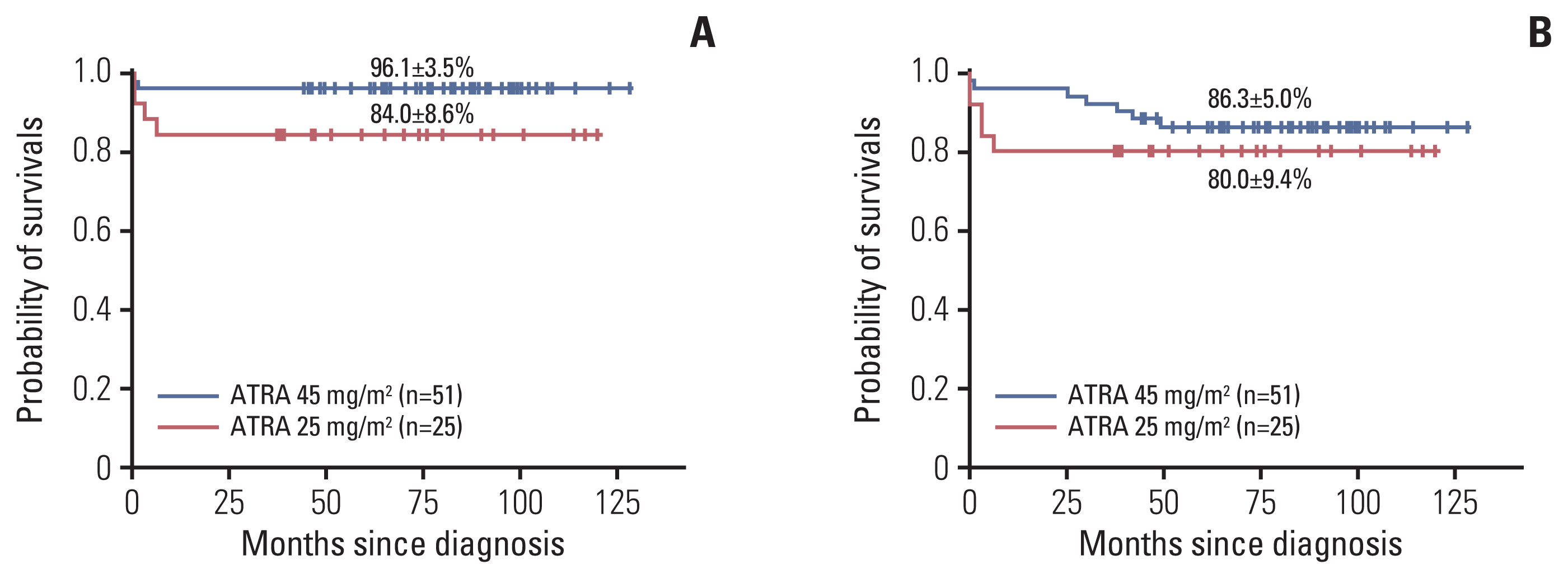
9]. In order to reduce these complications, studies on the use of low-dose ATRA were conducted, and one study reported that there was no difference between 25 and 45 mg/m
2/day of ATRA in the therapeutic efficacy and pharmacokinetic results [
17]. For this reason, many protocols for the treatment of pediatric APL have since used ATRA at a dose of 25 mg/m
2/day, and the treatment outcomes were reported to be similar to those who received conventional dose ATRA [
7,
9,
14]. In our study, the incidence of RA syndrome and headache was 55.3% and 28.9%, respectively, which was higher than reported in other studies. Specifically, the incidence of ATRA side effects was significantly higher in patients treated with 45 mg/m
2/day of ATRA than those treated with 25 mg/m
2/day of ATRA. But almost all cases of side effects including RA syndrome were transient, reversible, non-fatal and there was no significant difference in the OS and the EFS between groups according to ATRA dosage.
More recently, several studies reported that ATO therapy was effective and well-tolerated in pediatric APL [
8,
11,
18,
19]. Moreover, ATO could allow reduction of the overall amount of conventional chemotherapy and the cumulative dose of anthracyclines. In studies of relapsed pediatric APL patients, ATRA+chemotherapy or ATO alone have been reported to be effective in obtaining CR as a salvage therapy [
8,
18,
20]. Especially, ATO is well documented to induce second remission, in up to 80% of patients after two courses. Therefore, ATO containing regimens are the preferred salvage therapy [
11]. Five patients who relapsed in our study also successfully obtained CR with salvage treatment. Four patients received ATO with or without ATRA for induction therapy; it was tolerable and effective in obtaining second CR in relapsed patients. In addition, one patient who experienced second relapse after autologous HSCT achieved third CR after induction therapy with previously used ATRA alone.
Regardless of the agents used to induce second CR, HSCT is necessary to prevent further recurrence in relapsed APL children. Dvorak et al. [
21] analyzed the results of 32 pati-ents who received HSCT (11 autologous, 21 allogeneic) for relapsed or refractory APL. There was no statistical difference in survival rate between autologous and allogeneic HSCT. But, autologous HSCT was associated with a lower incidence of treatment-related mortality, while allogeneic HSCT was associated with a lower relapse rate. In our study, 1 (patient No. 1) out of two patients who received autologous HSCT experienced relapse again and eventually received an allogeneic HSCT from an human leukocyte antigen (HLA) matched sibling. All of the five relapsed patients are alive without evidence of disease. Like other types of leukemia, allogeneic HSCT may enhance survival because of the graft-versus-leukemia effect.
In conclusion, our study showed that the CR rate and OS in Korean pediatric APL patients were comparable to results that have been previously reported by study groups from North America and Europe. The initial WBC count was the most important prognostic factor in APL, and most of the causes of death were serious bleeding complications in the early stage of treatment. Low-dose ATRA was effective in reducing incidence of RA syndrome while resulting in survival outcomes comparable to conventional dose ATRA. In order to define other significant risk factors of APL in children, large scaled randomized prospective trials will be needed.
Go to :





 PDF
PDF Citation
Citation Print
Print


 XML Download
XML Download