Introduction
Materials and Methods
Results
Fig. 1
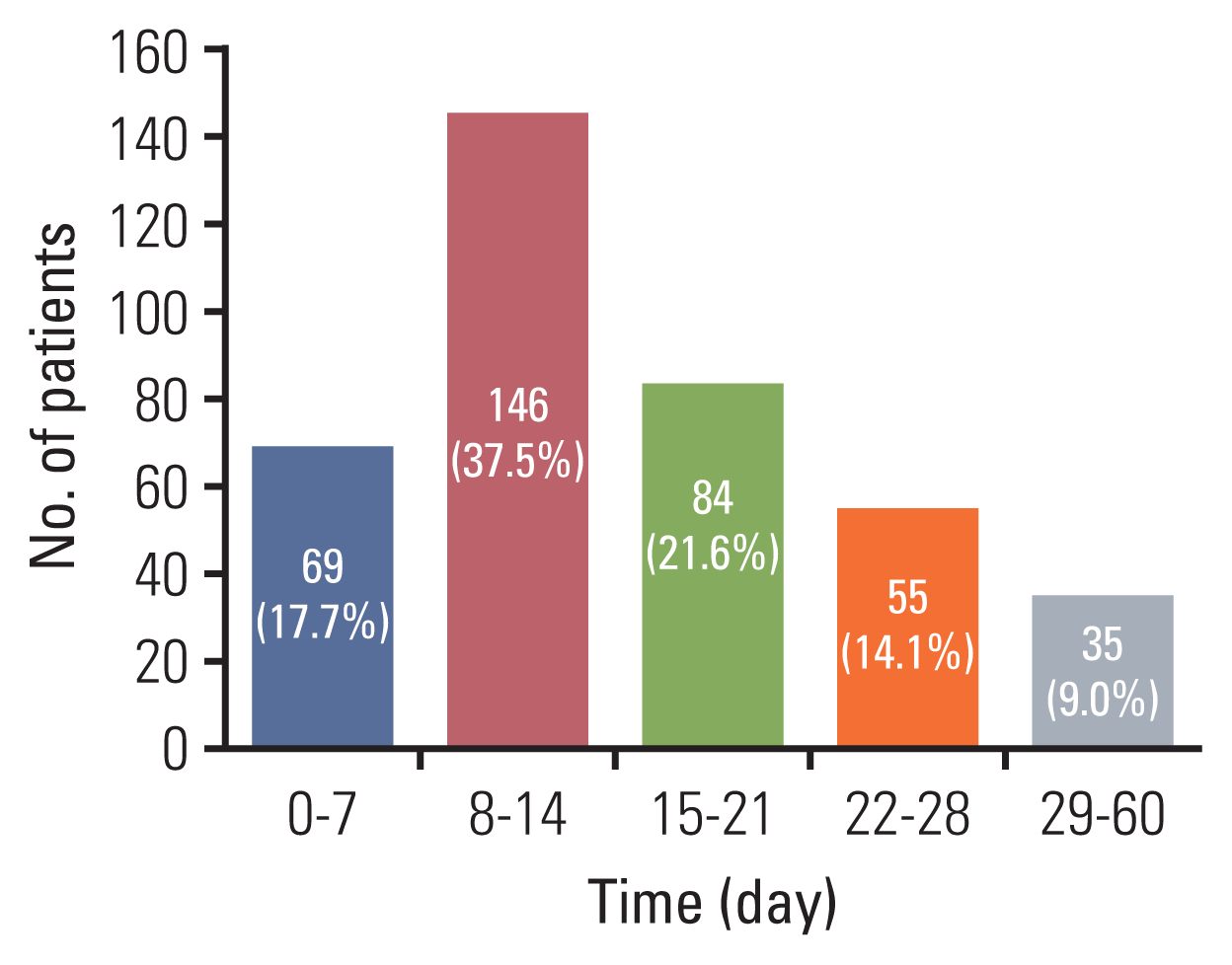
Table 1
| All (n=389) | Group 1 (≤ 14 days) (n=215) | Group 2 (> 14 days) (n=174) | p-value | |
|---|---|---|---|---|
| Intervals (day) | 14 (0–60) | 9 (0–14) | 22 (15–60) | < 0.001 |
| Age (yr) | 55 (25–85) | 53 (28–83) | 57 (25–85) | < 0.001 |
| FIGO stage | 0.066 | |||
| I | 31 (8.0) | 13 (6.0) | 18 (10.3) | |
| II | 205 (52.7) | 106 (49.3) | 99 (56.9) | |
| III | 83 (21.3) | 50 (23.3) | 33 (19.0) | |
| IV | 70 (18.0) | 46 (21.4) | 24 (13.8) | |
| Cell type | 0.152 | |||
| SCC | 337 (86.6) | 184 (85.6) | 153 (87.9) | |
| AD/ADS | 47 (12.1) | 30 (14.0) | 17 (9.8) | |
| Others | 5 (1.3) | 1 (0.5) | 4 (2.3) | |
| Chemotherapya) | 0.015 | |||
| Single agent | 224 (57.6) | 112 (52.1) | 112 (64.0) | |
| Combination | 165 (42.4) | 103 (47.9) | 62 (35.6) | |
| Early discontinuationb) | 0.525 | |||
| Yes | 40 (10.3) | 24 (11.2) | 16 (9.2) | |
| No | 349 (89.7) | 191 (88.8) | 158 (90.8) |
Fig. 2
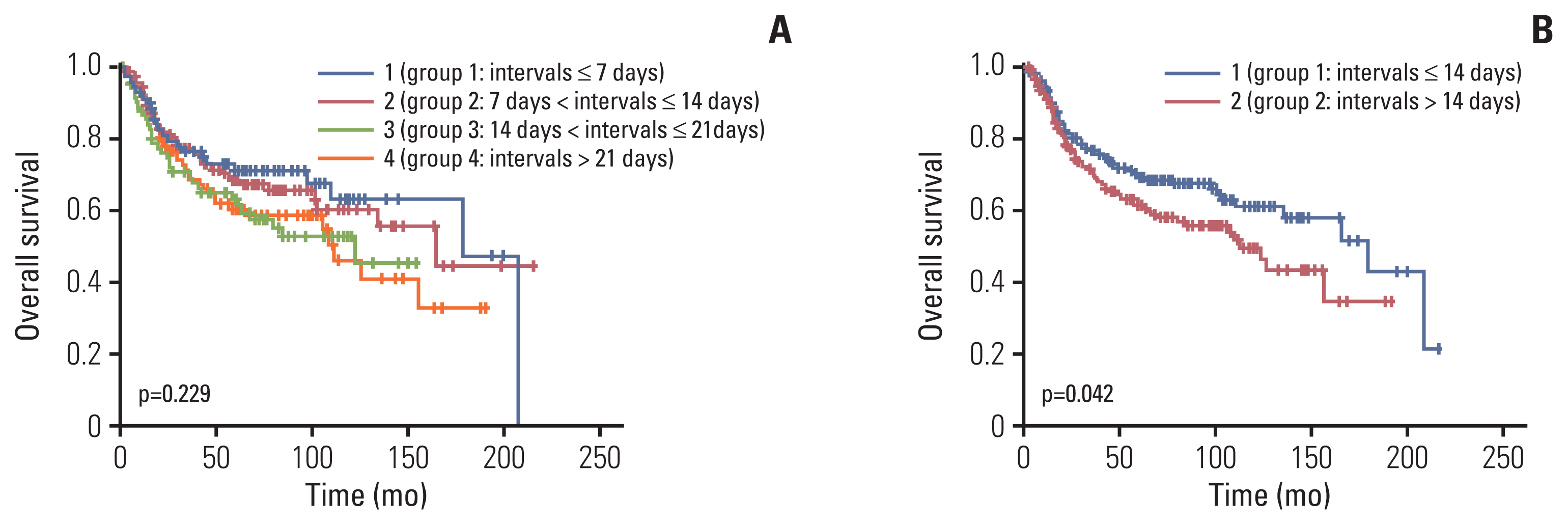
Table 2
| Univariable analysis | Multivariable analysis | |||||
|---|---|---|---|---|---|---|
|
|
|
|||||
| HR | 95% CI | p-value | HR | 95% CI | p-value | |
| Interval (day) | 1.019 | 1.003–1.036 | 0.020 | 1.023 | 1.006–1.040 | 0.007 |
|
|
||||||
| Age (yr) | 1.015 | 0.999–1.030 | 0.059 | 1.016 | 1.000–1.032 | 0.054 |
|
|
||||||
| FIGO stage | 0.001 | 0.001 | ||||
|
|
||||||
| I | 1 | 1 | ||||
|
|
||||||
| II | 1.102 | 0.528–2.304 | 0.796 | 1.146 | 0.543–2.417 | 0.721 |
|
|
||||||
| III | 2.015 | 0.941–4.316 | 0.071 | 2.211 | 1.028–4.759 | 0.042 |
|
|
||||||
| IV | 2.255 | 1.040–4.890 | 0.039 | 2.410 | 1.108–5.241 | 0.026 |
|
|
||||||
| Cell type | < 0.001 | < 0.001 | ||||
|
|
||||||
| SCC | 1 | 1 | ||||
|
|
||||||
| AD/ADS | 2.337 | 1.540–3.548 | < 0.001 | 2.148 | 1.410–3.273 | < 0.001 |
|
|
||||||
| Others | 4.550 | 1.440–14.372 | 0.010 | 5.855 | 1.802–19.031 | 0.003 |
|
|
||||||
| Chemotherapya) | ||||||
|
|
||||||
| Single agent | 1 | 1 | ||||
|
|
||||||
| Combination | 1.279 | 0.917–1.784 | 0.147 | 1.256 | 0.876–1.800 | 0.215 |
|
|
||||||
| Early discontinuatiob) | ||||||
|
|
||||||
| Yes | 1 | 1 | ||||
|
|
||||||
| No | 0.427 | 0.274–0.666 | < 0.001 | 0.426 | 0.270–0.671 | < 0.001 |
Discussion
Table 3
| Author | Year | No. | FIGO stage | Treatment | Definition of interval | Intervals (days) (No. of patients) | Effect on Survival |
|---|---|---|---|---|---|---|---|
| Perri et al. [18] | 1999–2010 | 321 | I–IV |
Surgery RT CCRT |
Bx to IT |
≤ 30 (134) 30–45 (86) > 45 (101) |
No effect on 3-year survival |
| Choan et al. [11] | 1991–2001 | 195 | IB–IV |
RT CCRT |
Bx to IT EUA to IT RO to IT |
24a) 21a) 21a) |
Adverse effect on DSS and OS |
| Ferreira da Silva et al. [9] | 2012–2014 | 865 | IA1–IVA |
Surgery RT CCRT |
Bx to IT Bx to TE |
11a) 274a) |
No survival outcome |
| Nascimento et al. [16] | 1995–2010 | 342 | I–IV |
RT CCRT |
Bx to IT Bx to RO RO to IT |
41a) 33a) 33a) |
No survival outcome |
| Umezu et al. [10] | 1999–2010 | 117 | IA–IIA | Surgery | IV to IT | 48a) | No effect on DFS and OS |
| Nanthamongkolkul and Hanprasertpong [19] | 1996–2012 | 441 | IA2–IB1 | Surgery | CS to IT |
≤ 8 ws (284) > 8 wk (157) |
Adverse effect on OS |
| Chen et al. [20] | 2004–2010 | 9,693 | I–IV |
Surgery RT CCRT |
Bx to IT |
≤ 90 (9,431) 90–180 (152) > 180 (200) |
Adverse effect on OS |
| Benard et al. [12] |
1996–2002 2003–2009 |
543 874 |
NA | NA | IV to Dx/Dx to IT |
33a)/22a) 21a)/19.5a) |
No survival outcome |
AT, additional therapy; Bx, biopsy; CCRT, concurrent chemoradiation; CS, clinical staging; DFS, disease free survival; DSS, disease specific survival; Dx, diagnosis; EUA, examination under anesthesia; FIGO, International Federation of Gynecology and Obstetrics; IT, initial therapy; IV, initial visit; NA, not available; OS, overall survival; RO, radiation oncology consultation; RT, radiotherapy; TE, therapy end time.




 PDF
PDF Citation
Citation Print
Print


 XML Download
XML Download