Introduction
Recent advances in imaging modalities and radiation therapy (RT) techniques have led to the improvement in survival and of locoregional control outcomes in patients with nasopharyngeal cancer (NPC) [
1]. However, the locoregional recurrence rate following definitive initial RT (iRT), with or without systemic chemotherapy, was reported to be 10%–36% [
2]. Salvage treatment for locally recurrent NPC has always been a difficult challenge. If managed with palliative treatment alone, very poor survival outcome approaching 0% 5-year survival rate is expected. Salvage surgery can be an option in selected patients who present with small and localized recurrence located within a safe focus; however, the procedure is not free from the risk of post-surgical morbidity and limited application in actual clinical situations [
3]. In this context, re-irradiation (re-RT) using up-to-date techniques has been frequently regarded as the best approach with salvage potential for most patients presenting with recurrence at or near the critical structures and for those with more advanced tumors, which usually impedes a safe and effective surgical approach [
3–
8]. However, re-RT has been frequently constrained by two important but disappointing factors: anatomical proximity to previously irradiated critical normal structures and possible presence of radiation resistance in recurrent tumors. These may require target delineation strategies and dose regimens that are different from the conventional approaches for successful local salvage. A recent meta-analysis established that re-RT by intensity-modulated radiation therapy (IMRT) can lead to the 5-year local failure-free survival (LFFS) and overall survival (OS) of 72% and 41%, respectively, which are associated with a 5-year fatal complication rate of 33% with minimal heterogeneity among the individual study reports [
9]. Because the survival benefit following IMRT re-RT is often offset by frequent fatal complications, careful planning and delivery of salvage re-RT is essential and highly recommended.
Hypo-fractionation was proposed to be more efficient in the treatment of tumors with heterogeneous radiation sensitivity by enhancing cell death and reducing the induction of resistance, compared with conventional fractionation schemes [
10]. A few studies have analyzed the fraction size to achieve a balance between the risk of toxicity and local control. Quan et al. [
11] reported that stereotactic ablative RT at a fractional dose of 5–9 Gy was a feasible option for the treatment of patients with recurrent head and neck cancer, which, however, could be limitedly applicable in cases of recurrent NPC as the target region is in close proximity to the surrounding critical structures.
The present study aimed to evaluate the clinical outcomes and safety of high-dose re-RT using moderate hypo-fractionation schemes in treating locally recurrent NPC patients.
Go to :

Materials and Methods
1. Patients
We retrospectively reviewed the records of 159 NPC patients who were registered to the Samsung Cancer Center database from 1995 till 2018 and presented with locoregional recurrence component following definitive iRT with or without systemic chemotherapy. To be eligible for the current study, the patients should (1) have had clinically evident local recurrence component including, retropharyngeal node (RPN) recurrence, but without distant metastasis component; and (2) have undergone hypo-fractionation re-RT, with or without chemotherapy, for salvage aim at our institute.
The patients with regional recurrence other than RPN without local recurrence component were excluded, and total of 60 patients formed the basis of the current analysis, among whom 30 (50.0%) received iRT at other hospitals and were referred to us for re-RT. The patients’ baseline characteristics are summarized in
Table 1. The median age was 53 years (range, 28 to 79 years) with a male preponderance (71.7%). As part of the initial workup, all patients underwent computed tomography (CT) or magnetic resonance imaging (MRI) scans of the head and neck, and
18F-fluorodeoxyglucose positron emission tomography-CT (PET-CT) scans were performed in 38 patients (63.3%). All patients showed evidently progressive lesion(s) on at least two consecutive imaging studies (CT, MRI, and PET-CT) during follow-up.
Table 1
|
Characteristic |
No. (%) |
|
Age, median (range, yr)
|
52.5 (28–79) |
|
Sex
|
|
Male |
43 (71.7) |
|
Female |
17 (28.3) |
|
ECOG PS
|
|
0–1 |
48 (80.0) |
|
2 |
12 (20.0) |
|
WHO histologic type at initial diagnosis
|
|
Keratinizing squamous cell carcinoma |
18 (30.0) |
|
Non-keratinizing carcinoma |
42 (70.0) |
|
Initial T category
|
|
iT1–2 |
32 (53.3) |
|
iT3–4 |
28 (46.7) |
|
Initial N category
|
|
iT0–1 |
30 (50.0) |
|
iT2–3 |
30 (50.0) |
|
Recurrent T category
|
|
rT1–2 |
31 (51.7) |
|
rT3–4 |
29 (48.3) |
|
Recurrent N category
|
|
rN0–1 |
56 (93.3) |
|
rN2–3 |
4 (6.7) |
|
Interval from initial RT to re-RT, median (range, mo)
|
26.5 (3–155) |

Histopathologic confirmation was done in 41 patients (68.3%), which was, however, impractical in other cases because of difficult anatomical location, such as the skull base. World Health Organization (WHO) histologic types were keratinizing squamous cell carcinoma in 18 patients (30.0%), and non-keratinizing carcinoma in 42 (70.0%), respectively. Anatomic stages were assigned according to the seventh edition of the American Joint Committee on Cancer (AJCC) system both for the initial and recurrent stages. The initial stages were iT1–2 in 32 patients (53.3%) and iT3–4 in 28 (46.7%), and iN0–1 in 30 (50.0%) and iN2–3 in 30 (50.0%), respectively. The recurrent stages were rT1–2 in 31 patients (51.7%) and rT3–4 in 29 (48.3 %), and rN0–1 in 56 (93.3%) and rN2–3 in four (6.7%), respectively. The median interval between the last day of iRT and the first day of re-RT (disease-free interval [DFI]) was 26.5 months (range, 3 to 155 months).
2. Initial radiation therapy
The treatment characteristics are summarized in
Table 2. The iRT techniques of 2-/3-dimensional (2D/3D) RT were used in 31 patients (51.6%), while IMRT was performed in 29 patients (48.3%). The median total iRT dose was 70.0 Gy (range, 48.0 to 86.0 Gy) with the median fraction size of 2.0 Gy (range, 1.8 to 2.5 Gy). The median biological effective doses (BEDs) for the early responding tissues (assuming α/β ratio=10, BED
10) and the late responding tissues (assuming α/β ratio=3, BED
3) were 84.0 Gy (range, 57.6 to 107.2 Gy) and 116.7 Gy (range, 80.0 to 156.5 Gy), respectively. The policy of target delineation and dose prescription in iRT at our institute was described previously [
12]. The median gross tumor volume (GTV) of re-RT, excluding nodal target volume, was 6.0 mL (range, 1.0 to 89.0 mL). The majority of the patients received cisplatin-based chemotherapy in addition to iRT: concurrent chemotherapy (CCRT) in 41 patients (68.3%); and induction chemotherapy in 12 (20.0%), respectively.
Table 2
Treatment characteristics
|
Characteristic |
Initial RT |
Re-RT |
|
RT modality
|
|
2D-RT |
11 (18.3) |
0 |
|
3D-CRT |
20 (33.3) |
20 (33.3) |
|
IMRT |
29 (48.3) |
33a) (55.0) |
|
IMPTb)
|
0 |
7 (11.7) |
|
RT fraction size (range)
|
|
1.8 Gy |
24 (40.0) |
0 |
|
2.0 Gy |
18 (30.0) |
0 |
|
2.15–2.46 Gy |
18 (30.0) |
4 (6.7) |
|
2.5 Gy |
0 |
24 (40.0) |
|
3.0 Gy |
0 |
29 (48.3) |
|
3.2–4.0 Gy |
0 |
3 (5.0) |
|
Median (range, Gy) |
2.0 (1.8–2.5) |
3.0 (2.3–4.0) |
|
Total RT dose
|
|
BED10 (Gy) |
84.0 (57.6–107.2) |
78.0 (56.0–87.5) |
|
BED3 (Gy) |
116.7 (80.0–156.5) |
120.0 (91.7–132.3) |
|
Median (Gy) |
70.0 (48.0–86.0) |
60.0 (40.0–70.0) |
|
Cumulated RT dose
|
|
BED10 (Gy) |
160.2 (135.6–194.7) |
|
|
BED3 (Gy) |
230.1 (200.0–284.9) |
|
|
Additional chemotherapy
|
|
Not done |
7 (11.7) |
47 (78.4) |
|
Induction chemotherapy |
12 (20.0) |
5 (8.3) |
|
Concurrent chemotherapy |
41 (68.3) |
8 (13.3) |

3. Re-irradiation
The re-RT techniques actually used were 3-dimensional conformal radiation therapy (3D-CRT) in 20 patients (33.3%), IMRT throughout the course of re-RT in 33 (55.0%), and intensity-modulated proton beam therapy (IMPT), either in combination with IMRT or as a sole modality, in seven (11.7%). For patients treated with IMPT, the pencil beam scanning technique was used with the relative biological effectiveness (RBE) set at 1.1. This difference in the RT techniques was partly attributed to the changes in the Korean National Health Insurance System (KNHIS) reimbursement policy, which began to cover IMRT since 2012. IMPT has been actively implemented clinically at our institute in December 2015.
The principles of target volume delineation and dose constraints of the organs at risk were identical throughout the study period. No elective nodal irradiation was administered, and the clinical target volume consisted of the GTV plus a patient-specific margin (0.5–1.5 cm), which depended mainly on the tumor location and proximity of the adjacent organs at risk. The doses delivered to the normal organs were not constrained, but an effort was made to avoid the hotspots. The median total dose of re-RT was 60.0 Gy (range, 40.0 to 70.0 Gy) with a median fraction size of 3.0 Gy (range, 2.3 to 4.0 Gy). The median BED10 and BED3 were 78.0 Gy (range, 56.0 to 87.5 Gy) and 120.0 Gy (range, 91.7 to 132.3 Gy), respectively. The median cumulative BED10 and BED3 were 160.2 Gy (range, 135.6 to 194.7 Gy) and 230.1 Gy (range, 200.0 to 284.9), respectively. The majority of patients (47 patients, 78.4%) did not receive additional chemotherapy along with re-RT, while eight (13.3%) received CCRT, and five (8.3%) did induction chemotherapy.
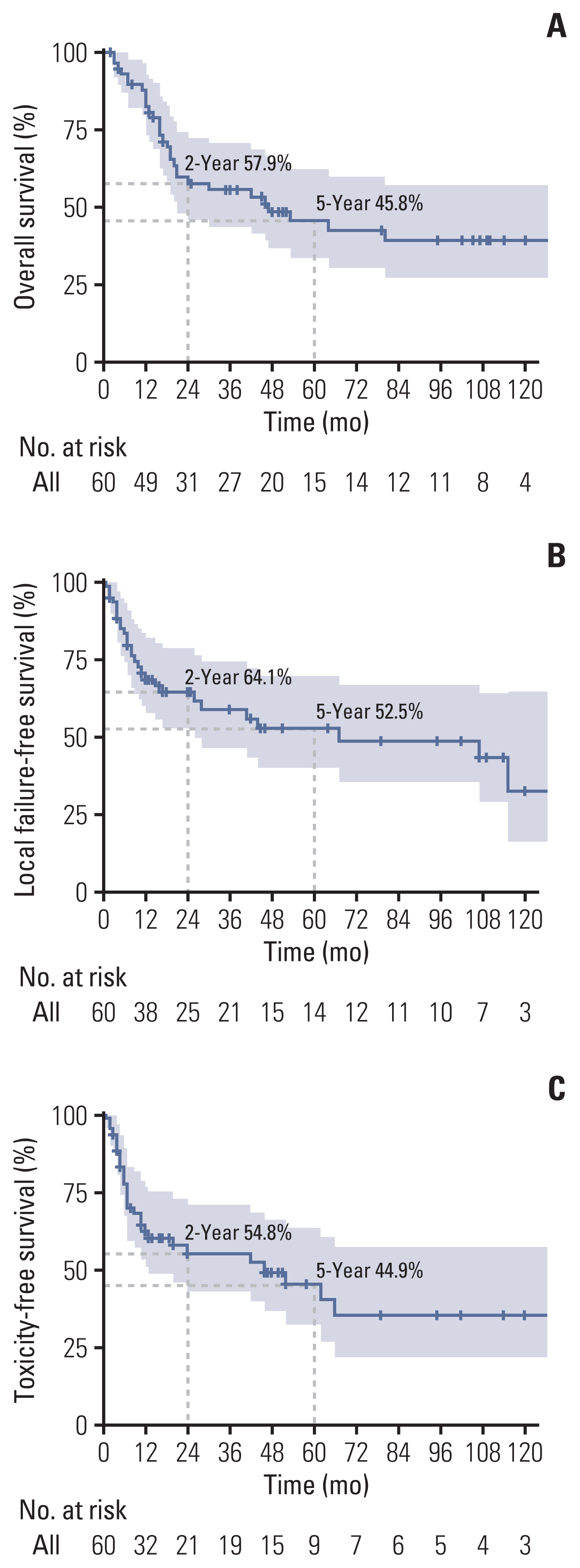
4. Patients’ evaluation and follow-up
During the re-RT course, all patients were examined weekly to evaluate the acute toxicity profiles and tumor response. Tumor response was assessed by CT in 1 month and PET-CT in 4 months of re-RT completion, respectively. Subsequent regular follow-up’s were carried out, using appropriate imaging study (CT and/or MRI), at every 3–4 months’ interval during the first 2 years and every 6–12 months’ interval thereafter. Local re-recurrence was defined as persistence, progression or reappearance of the irradiated lesion(s), or appearance of new lesion(s) within the re-RT target volume. A biopsy was attempted to confirm local failure whenever necessary and feasible. Treatment-related toxicities were scored according to the Common Terminology Criteria for Adverse Events, ver. 5.0. The dates of the first appearance of non-preexisting symptoms/signs unrelated to the tumor itself were recorded and used to calculate the toxicity-free survival (TFS). In patients with toxicities in multiple organs, the highest grade of toxicity per patient was used for analysis.
5. Statistical analyses
The survival outcomes were calculated using the Kaplan-Meier method, and the durations of OS, LFFS, and TFS were measured from the first date of re-RT course. The Fisher’s exact test and chi-square test were used to compare the effects of prognostic factors on the outcomes. A correlation analysis between dose parameters was performed using the Spearman’s method. Univariate and multivariate analyses were carried out using the log-rank test and the Cox proportional hazards model with stepwise selection of variables. The dose, fractionation, and BED were analyzed separately in the multivariate analysis. R ver. 3.6.3 (R Foundation, Vienna, Austria) was used for all statistical analyses, and a p-value of less than 0.05 was considered significant.
Go to :

Discussion
Several previous investigators attempted to determine the optimal re-RT dose schedule to obtain maximal clinical benefits. In one study that included 91 re-RT patients [
7], who were initially treated with IMRT, the OS rate peaked at approximately 60 Gy
10 (EQD2), however, the local control rate flattened out, the fatal late complication rate increased, and the OS rate declined beyond 60 Gy
10. A recent meta-analysis of re-irradiation for NPC [
9] concluded that the total dose of at least 60 Gy was required to achieve adequate local control. The maximum safe re-RT dose remained still unknown, and the GTV mean dose of ≥ 70 Gy was not associated with further improvement in local control or OS in their analysis.
In modern re-RT series, IMRT or particle beam therapy, such as proton or carbon ion beams, seems advantageous in delivering radiation doses of > 60 Gy while minimizing the risk of normal organ damage. Zhang et al. [
13] reported a significantly improved therapeutic ratio when using IMRT, compared with 2D- or 3D-RT, in a meta-analysis including 3,570 patients. In a recent study on 251 locally recurrent NPC patients, who underwent IMRT re-RT, Li et al. [
14] reported that EQD2 of 68 Gy or higher was associated with significantly worse OS. IMRT has been the most commonly employed RT technique in the treatment of NPC patients in Korea since 2010, and 48.3% of the patients in the current study underwent IMRT as iRT, who achieved better OS with borderline significance in multivariate analyses (hazard ratio [HR], 0.45; 95% confidence interval [CI], 0.20 to 1.02; p=0.055).
A few studies reported the outcomes following re-irradiation using proton or carbon ion beam therapy. A historical study by Lin et al. [
15] reported 50% of both OS and locoregional progression-free survival at 2 years in 16 recurrent NPC patients who were treated by proton beam re-RT (median, 60.1 Gy RBE), with minimal side effects to the central nervous system. Dionisi et al. [
5], on the other hand, reported their proton beam re-RT experience to 17 patients (12 with rT4) with the median 10 months’ follow-up: grade ≥ 3 late toxicities occurred in 23.5% of the patients, while fatal bleeding of uncertain cause (either tumor recurrence or carotid blowout) occurred in one; and 18-months’ rates of OS and local control were 54.4% and 66.6%, respectively. Hu et al. [
16] reported the outcomes of 75 recurrent NPC patients (all had poorly or undifferentiated carcinoma, and 36% presented with rT4 disease) who received carbon ion beam re-RT (50–66 Gy RBE). After the median follow-up of 15.4 months, 1-year LFFS rate was 87%, and mucosal necrosis and temporal lobe necrosis developed in 9.3% and 1.3% of the patients, respectively. These results following particle beam therapy as re-RT modality are, more or less, favorably comparable to those following photon-based RT techniques, and further studies with enough sample size and longer-term observation would be needed.
Considering the tumor cell heterogeneity within recurrent tumors and probable presence of radiation-resistant clones, a few studies have been conducted to identify the optimal and biologically efficacious dose schedules. Based on the biological model in one study [
10], increasing the daily dose and decreasing the total treatment time is assumed to result in higher tumor killing without developing predominant radiation-resistant clone, and we hypothesized that hypo-fractionation strategy, under the equivalent BED, could induce radiation resistance less frequently when compared with conventional fractionation dose scheme. Previous literatures reported superior outcomes with the increased fraction size in glottis cancer patients [
17,
18]. Nevertheless, the use of hypo-fractionation has been limited in fear of the concomitant increased risk of normal tissue damage, in re-RT setting where the target is surrounded by the critical neural structures as in re-RT for recurrent NPC.
Table 5 summarized several previous studies and the current one which applied hypo-fractionation re-RT in treating the recurrent NPC patients for easy comparison. Wide range of hypo-fractionation regimen was applied by several previous studies, and the fractional dose was typically larger in those using stereotactic RT technique. The largest series by Lee et al. [
19] reported that 5-year actuarial rates of complication-free survival and OS were 48% and 12% among 485 patients who underwent re-RT by classic techniques with the median fractional dose of 3 Gy (range, 1.8 to 5 Gy). Tian et al. [
20] prospectively compared 68 Gy in 34 fractions (2 Gy per fraction) vs. 60 Gy in 27 fractions (2.22 Gy per fraction) using IMRT through a phase II trial. Though there were no statistical differences in survival and local control outcomes, however, the incidence rates of mucosal necrosis and massive hemorrhage, which were the primary causes of death, were significantly higher in the 2 Gy per fraction group than those in the 2.2 Gy per fraction group (50.8% vs. 28.8%; HR, 2.3; 95% CI, 1.1 to 5.0; p=0.02). In the study by Hu et al. [
16], re-RT series using carbon ion therapy, 43 of 75 patients were irradiated using a fraction size of 3 Gy RBE. On univariate analysis, dose of 3 Gy RBE per fraction (compared with smaller fraction sizes) and a BED of ≥ 74.1 Gy RBE at re-RT significantly improved the progression-free survival. There were three studies that employed larger fraction sizes by stereotactic RT technique. Leung et al. [
21] used fractionated dose schedules to 30 patients to deliver the median total dose of 54 Gy (range, 32 to 57 Gy) in median 18 fractions (range, 8 to 22) by 2.5–4.5 Gy per fraction. Nine out of 30 patients had rT3–4 stage tumors, and the OS and major complication-free rates at 5 years were 40% and 21.4%, respectively. Ozyigit et al. [
22] reported 2-year local control rate of 82% with fatal complication rate of 12.5% in 24 patients who received the total dose of 30 Gy in 5 fractions. Seo et al. [
23], following total dose of 24–45 Gy in 3–5 fractions by fractional dose of 7.5–12.0 Gy, reported that the 5-year rates of OS and LFFS were 60% and 79%, respectively, while three and two patients (9.4% and 6.3%) developed grade 4 and 5 toxicities, respectively. In our study, the clinical outcomes including survival and toxicity were comparable with the previous studies, and cumulative dose or fraction size ≥ 3 Gy at re-RT showed no significance on OS, LFFS, and TFS.
Table 5
Summary of previous studies for hypo-fractionation re-RT in locally recurrent nasopharyngeal cancer patients, and comparison with the outcomes of the current study
|
No. of patients |
1st RT modality |
Re-RT modality |
Total dose for re-RT (Gy) |
Fraction size for re-RT (Gy) |
Survival |
Major morbidity |
|
Lee et al. (2000) [19] |
487 |
2D RT |
2D RT |
45.6 (7.5–70) |
3 (1.8–5) |
5-Year OS, 12% |
5-Year TFS, 48% |
|
Ng et al. (2020) [7] |
91 |
IMRT |
3D RT, 1.1%
IMRT, 83.5%
SBRT, 15.4% |
60 (45–70) |
1.2 (bid), 19.8%
1.8–2.5, 63.7%
> 2.5, 16.5% |
2-Year OS, 59.6% |
18.7% |
|
Tian et al. (2014) [20] |
58 |
IMRT, 1.7% |
IMRT |
60 |
2.2 |
5-Year OS, 42.2% |
28.8% |
|
Hu et al. (2018) [16] |
75 |
IMRT, 96% |
Carbon ion therapy |
50–66 |
2–3 |
1-Year OS, 98.1% |
Grade 3–4, 11.9% |
|
Leung et al. (2009) [21] |
30 |
2D/3D RT |
SBRT |
54 (32–57) |
3 (2.5–4.5) |
5-Year LFFS, 56.8%
5-Year OS, 40% |
5-Year major TFS, 21.4% |
|
Ozyigit et al. (2011) [22] |
24 |
N/A |
SBRT (Cyberknife) |
30 |
6 |
2-Year CSS, 64%
2-Year LFFS, 82% |
12.5% |
|
Seo et al. (2009) [23] |
35 |
N/A |
SBRT (Cyberknife) |
24~45 |
7.5–12 |
5-Year OS, 60% |
Grade 4–5, 6% |
|
Current study |
60 |
2D/3D RT 51.7%
IMRT, 48.3% |
3D RT, 33.3%
IMRT/IMPT, 66.7% |
60 (40–70) |
3.0 (2.3–4.0) |
5-Year LFFS, 52.5%
5-Year OS, 45.8% |
5-Year grade ≥ 3
TFS, 84.7%
Grade 5, 13.1% |

In our study, histologic type had an impact on LFFS, as well as rT and rN categories, as shown in the multivariate analysis, and local control in keratinizing squamous cell carcinoma seemed worse. The WHO subtypes squamous cell carcinoma of the nasopharynx as non-keratinizing, keratinizing and basaloid squamous cell carcinoma. Non-keratinizing tumors are further subcategorized into undifferentiated or differentiated types [
24]. The proportion of keratinizing type was less than 5% in high-incidence regions [
25], and this subtype was less sensitive to RT and more frequently associated with local failure than the non-keratinizing type [
26,
27]. In our study, the proportion of keratinizing tumors was larger (30.0%) than that of most published re-irradiation series, which were mainly from the high-incidence regions. Considering the relative radiation resistance of keratinizing tumors, especially recurrent tumors, further studies on differential target delineation and dose schedule based on histologic type are warranted.
Recently, international consensus recommendations on re-RT for recurrent NPC has been published [
28], in which 29 expert panels from 25 different institutes (in 14 different countries) participated. Most of the important issues, including from the clinical indications to the details of RT, were covered and summarized through the multi-step voting by the expert panels. As for the dose fractionation issue, majority of the international expert panels recommended hyper-fractionation schedule (71%), followed by once daily fractionation (29%). Hypo-fractionation schedule of using 2.5 Gy or 3.0 Gy per fraction, when using once daily fractionation, was also recommended by 4 panels (17%). We have applied moderate hypo-fractionation schedules to the NPC patients, not only in re-RT but also in iRT settings, since over 10 years ago. Our policy of moderate hypo-fractionation has been in concordance with the limited hardware resource when compared to the departmental patients’ load at our institute, where twice daily fractionation schedule is not feasible at all, and is somewhat different from the international recommendations. This consensus strongly endorsed high-dose re-RT by IMRT techniques. There were several favorably comparable reports on particle beam re-RT, and 82% of the panels suggested considering particle beam therapy if available. It was also advised to undergo careful comparison of the RT planning by IMRT and particle beam therapy, which has been our policy before the actual assignment of RT modality to each patient since after we started proton beam therapy. However, the advanced techniques were not always associated with improved outcomes. Li et al. [
29] developed a nomogram to predict post-RT mucosal necrosis based on over 7,000 NPC patients who underwent RT. In addition to several clinical and baseline nutritional and inflammatory status of the patients, they found two important RT-related adverse factors: re-RT; and IMRT (when compared with 2D RT), respectively. Recently, we investigated the clinical features of 22 patients who developed post-RT mucosal necrosis, and also confirmed Li et al.’s report [
29]. With the advent of RT technique, coupled with frequent application of CCRT and moderate hypo-fractionation (as in simultaneous integrated boost), the overall trend has evidently been toward the increased aggressiveness in treating the NPC patients. In the current study, rIMRT/IMPT was one of the adverse factors for TFS. We noticed that, during re-RT, rIMRT/IMPT patients received CCRT (20% vs. 0, p=0.043) and fractional dose > 3.0 Gy (62.5% vs. 35%, p=0.058) more frequently. It is not infrequent to observe the competitive interrelationship between the causes of major morbidity and/or death in cancer patients: cancer recurrence itself vs. treatment-related toxicity. Following the aggressive approach, however, we could achieve improved survival outcomes and observe more frequent major toxicity events, which could be translated into so-called “survivorship bias”. Nevertheless, more enthusiastic efforts to build the objective evidences and to develop optimal guidelines in utilizing the advanced RT techniques should be carried out and encouraged, which do not limit to IMRT but to include particle beam therapy.
The current study has two limitations: the study was based on not big enough number of patients; and one-third of them were treated with 3D-CRT. The incidence of nasopharynx cancer in Korea is not very high (roughly about 400 new patients per year), so is that of local recurrence events, which resulted in not enough sample size though the study duration was quite long (1995–2018). When considering that Korea is not endemic region of NPC, this sample size seems not too small to reflect the real world situation in Korea. Likewise, the RT modalities used in the current study were not uniform throughout longer than 20 years’ time period. 3D-CRT technique that we actually used was represented as fractionated stereotactic RT, which routinely included target delineation with tight margins, and daily immobilization and target location verification procedures with non-invasive frame. This technique was our main re-RT modality until before KNHIS recognized IMRT as a new RT technique and began its reimbursement. However, the current study has the advantage of employing modest hypo-fractionation schedule in all patients, in an effort to overcome the problems of limited resource and the issue of possible hypoxia and radiation resistance, which enabled the investigation of the impact of this dose schedule on the outcomes. We previously reported our experience of 21 locally recurrent NPC patients, all of who underwent 3D-CRT as re-RT technique [
30]. The median total dose was 55 Gy, either at 2.5 Gy or 3.0 Gy per fraction, and the 5-year OS rate was 32.3%; meanwhile, three patients (14.3%) developed fatal complications (massive epistaxis, temporal lobe necrosis, and brainstem necrosis). The current study included additional patients, and most of them were treated with IMRT as iRT and IMRT (with or without or IMPT) as re-RT modality. Following the consistent policies of target delineation and dose prescription throughout the entire study period, the 5-year OS rate improved (45.8%) with a similar incidence of fatal complications (seven events, 11.7%), when compared with the previous study. In addition, the current study demonstrated that the re-RT technique was significantly associated with 2-year OS rate (40.0% following 3D-CRT vs. 68.5% following IMRT/IMPT, p=0.012) (
Table 3), which partly indicated that the temporal changes in RT techniques were associated with significantly improved clinical outcomes.
Overall, 60 patients had recurrent NPC, among whom 46.7% had rT3–4 tumors and 30.0% had keratinizing squamous cell carcinoma. We could, following re-RT with moderate hypo-fractionation dose schedule, achieve similar clinical outcomes (5-year OS and LFFS rates of 45.8% and 52.5%), with less frequent fatal complications (cumulative grade 5 toxicity event rate of 11.7%), when compared with the previous studies, mainly from endemic countries. Both survival and toxicity profiles were largely affected by the characteristics of iRT. Re-RT by IMRT (with or without IMPT) with a moderate hypo-fractionation dose schedule seemed feasible, based on the comparable outcome profiles. Further studies with a larger sample size are warranted to confirm our findings.
Go to :





 PDF
PDF Citation
Citation Print
Print


 XML Download
XML Download