Introduction
Materials and Methods
1. Chemicals
2. Cell culture
3. Cell viability assay
4. Western blot analysis
5. Quantification of apoptotic cell death by flow cytometry
6. Cell migration assay
7. Immunofluorescence and immunohistochemistry
8. In vivo xenograft model
9. Statistical analysis
Results
1. Cell viability after docetaxel and Ku-0063794 mono- and combination therapy
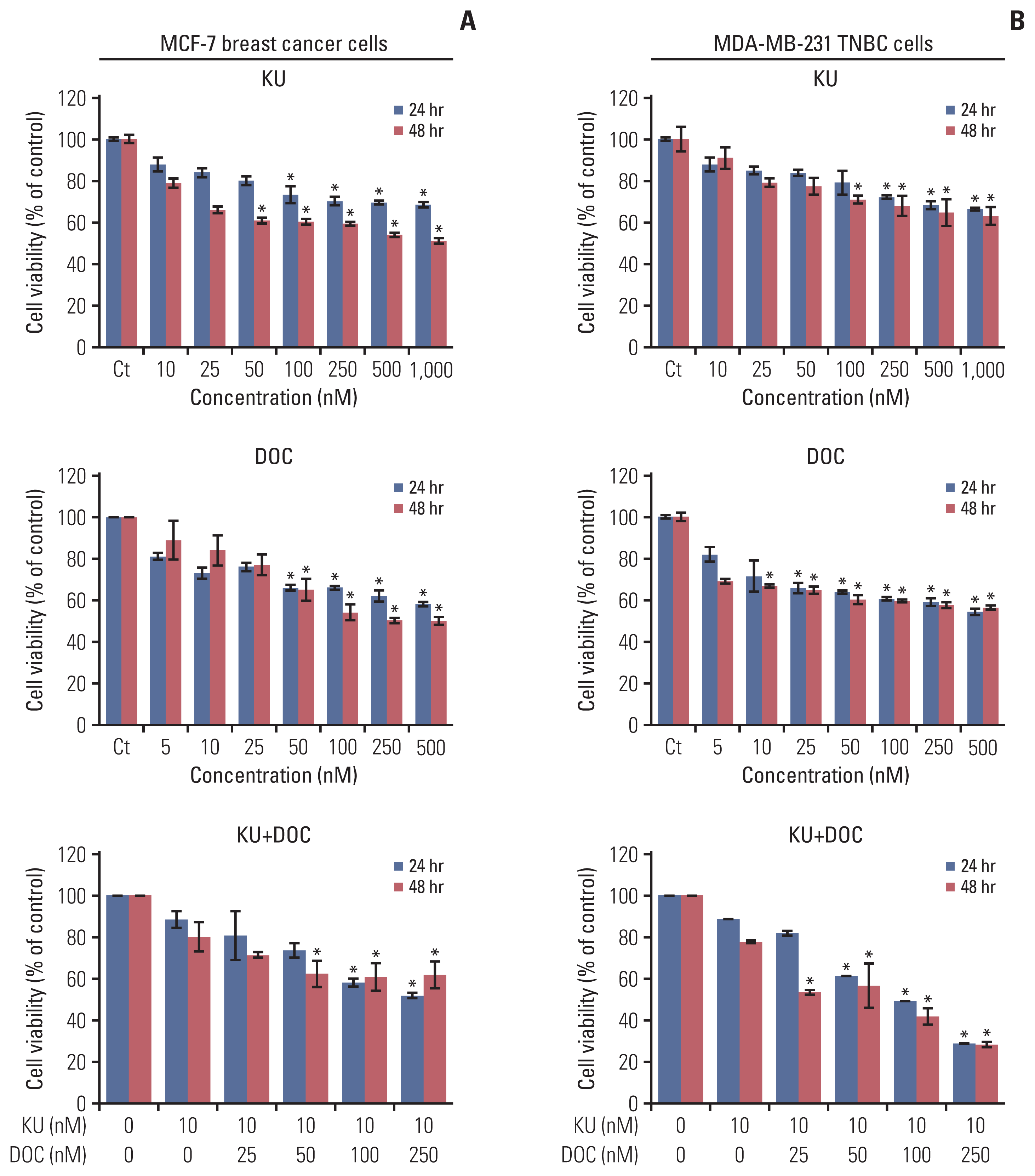 | Fig. 1Cell viability tests of breast cancer cells following docetaxel and Ku-0063794 mono- and combination therapy. (A) Viability of MCF-7 human breast cancer cells according to increasing concentration of Ku-0063794, docetaxel, and their combination. (B) Viability of MDA-MB-231 triple-negative breast cancer (TNBC) cells according to increasing concentration of Ku-0063794, docetaxel, and their combination. DOC, docetaxel; KU, Ku-0063794. Values are presented as mean±standard deviation of three independent experiments. *p < 0.05. |
2. Apoptosis following mono- and combination therapies
 | Fig. 2Cell apoptosis following docetaxel (DOC) and Ku-0063794 (KU) mono- and combination therapies. (A) Western blot analysis showing the effects of mono- and combination therapy on apoptosis of MCF7 breast cancer cells. Relative densities of individual markers. The relative densities had been quantified using Image J software and then were normalized to the density of β-actin in each group. (B) Western blot analysis showing the effects of mono- and combination therapy on apoptosis of MDA-MB-231 triple-negative breast cancer (TNBC) cells. Relative densities of individual markers. (C) Quantitative analysis of the effects of KU and DOC combination therapy on the apoptosis of MCF-7 breast cancer cells using Annexin V/propodium iodide (PI) staining and flow cytometry. Apoptotic cells were expressed as the total percentage of Annexin V-positive/PI-negative cells. (D) Quantitative analysis of the effects of KU and DOC combination therapy on the apoptosis of MDA-MB-231 TNBC cells using Annexin V/PI staining and flow cytometry. The total percentage of Annexin V–positive/PI-negative cells. Mcl-1, myeloid cell leukemia 1; PARP, poly-ADP (adenosine diphosphate)-ribose polymerase. Values are presented as mean±standard deviation of three independent experiments. *p < 0.05. |
3. Epithelial-mesenchymal transition and cell migration following mono- and combination therapies
 | Fig. 3Effects of docetaxel (DOC) and Ku-0063794 (KU), either individually or in combination, on epithelial-mesenchymal transition (EMT) and migration of breast cancer cells. (A) Western blot analyses showing the expression of EMT-related markers in MCF-7 breast cancer cells following mono- and combination therapies of DOC and KU. Relative densities of individual markers. The relative densities had been quantified using Image J software and then were normalized to the density of β-actin in each group. (B) Western blot analyses showing the expression of EMT-related markers in MCF-7 and MDA-MB-231 triple-negative breast cancer (TNBC) cells following mono- and combination therapies of DOC and KU. Relative densities of individual markers. (C) Wound healing assay (×200, scale bar=20 μM) showing the effects of DOC and KU, either individually or in combination, on the migration of MCF-7 and MDA-MB-231 breast cancer cells. Migration was expressed as percentage of cells migrated compared to the control. Values are presented as mean±standard deviation of three independent experiments. *p < 0.05. |
4. Changes in autophagic markers following mono- and combination therapies
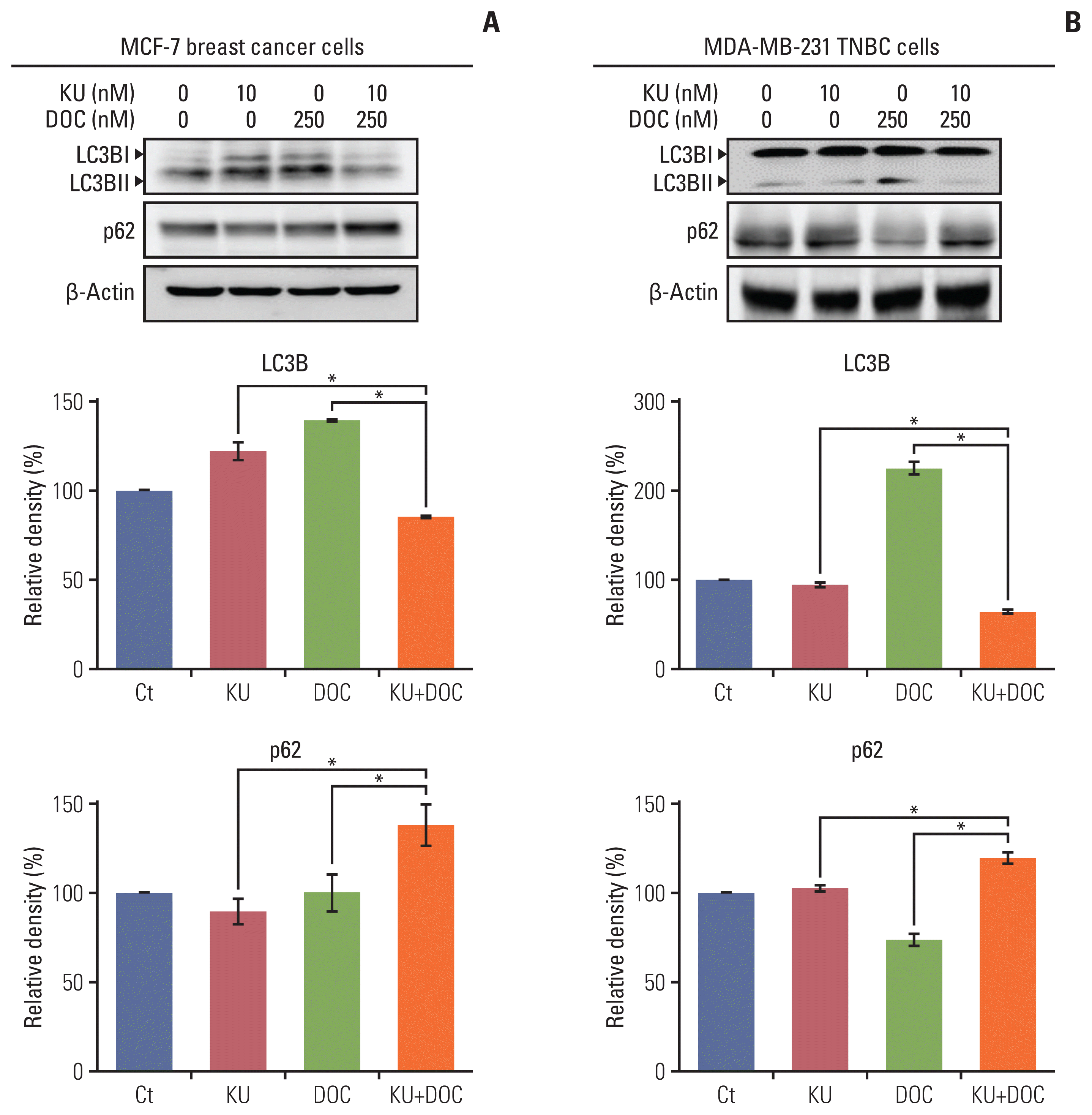 | Fig. 4Effects of docetaxel (DOC) and Ku-0063794 (KU), either individually or in combination, on the autophagy of breast cancer cells. (A) Western blot analysis showing the expression of autophagy-related markers in MCF-7 breast cancer cells following mono- and combination therapies of DOC and KU. Relative densities of individual markers. The relative densities had been quantified using Image J software and then were normalized to the density of β-actin in each group. (B) Western blot analysis showing the expression of autophagy-related markers in MDA-MB-231. TNBC cells following mono- and combination therapies of DOC and KU. Relative densities of individual markers. LC3B, microtubule-associated proteins 1A/1B light chain 3B. Values are presented as mean±standard deviation of three independent experiments. *p < 0.05. |
5. Validation of combination therapy anticancer effects in vivo
 | Fig. 5Effects of docetaxel (DOC) and Ku-0063794 (KU), individually and in combination, on the growth of MCF-7 and MDA-MB-231 cells xenografted into nude mice. After DOC (1 mg/kg/day) and KU (1 mg/kg/day) had been administered intraperitoneally three times a week for 3 weeks, the mice were euthanized, and the tumors were collected. (A) Morphological images of mice with xenografted MCF-7 and MDA-MB-231 cells following mono- and combination therapies of DOC and KU. Images of tumors after necropsy show that tumor shrinkage was more prominent in mice treated with combination therapy than in the mice treated with individual monotherapies. (B) Comparison of tumor size over time after injecting DOC and KU, either individually or in combination, into MCF-7 and MDA-MB-231 cells over time, respectively. In both types of breast cancer cells, a considerable reduction in tumor size was observed in mice treated with combination therapy than in mice treated with individual monotherapies. (C) Hematoxylin and eosin stains (top left) and cleaved caspase-3 (top middle) and Bcl-xL (top right) immunohistochemical stains of the MDA-MB-231 cells xenografted in nude mice after injecting docetaxel (DOC) and Ku-0063794 (KU), either individually or in combination. Percentages of cell count (bottom left) and immunoreactive areas (bottom middle and right) were measured using Image J and expressed as relative to the control. Values are presented as mean±standard deviation of three independent experiments. *p < 0.05. |
6. Comparison of each group by tissue staining
7. Validation of autophagy inhibition by combination therapy in vivo
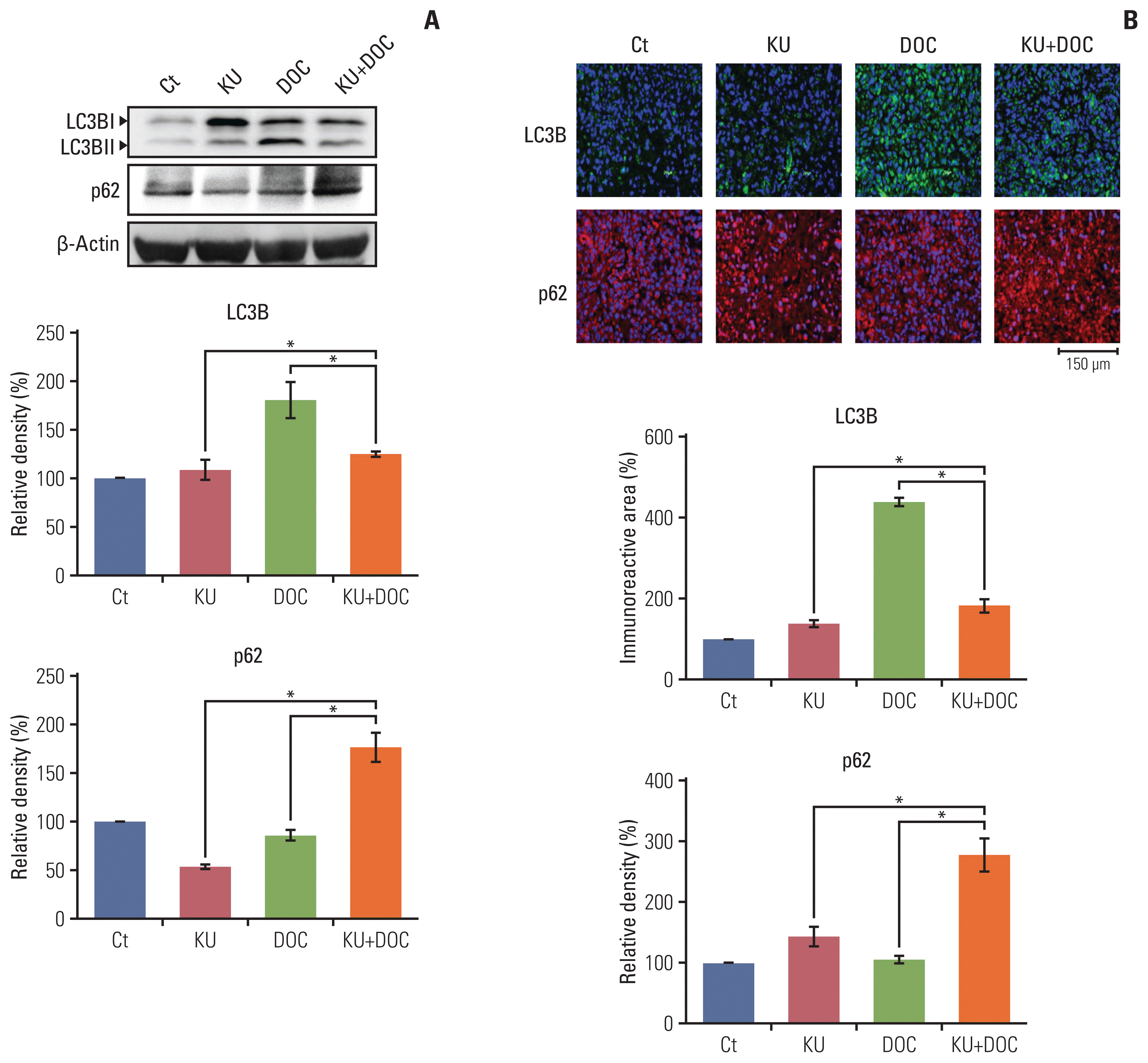 | Fig. 6Effects of each treatment on autophagy of triple-negative breast cancer (TNBC) cells in vivo. (A) Western blot analysis showing the expression of autophagy-related markers in MDA-MB-231 TNBC cells xenografted in nude mice following docetaxel (DOC) and Ku-0063794 (KU), mono- and combination therapies. Relative densities of LC3B and p62 proteins. The relative densities had been quantified using Image J software and then were normalized to the density of β-actin in each group. (B) LC3B and p62 immunofluorescence of MDA-MB-231 TNBC cells xenografted in nude mice following DOC and KU mono- and combination therapies. Percentages of immunoreactive areas were measured using NIH image J and expressed as relative values to the control. LC3B, microtubule-associated proteins 1A/1B light chain 3B. Values are presented as mean±standard deviation of three independent experiments. *p < 0.05. |
8. Validation of the effects of combination therapy on EMT in vivo
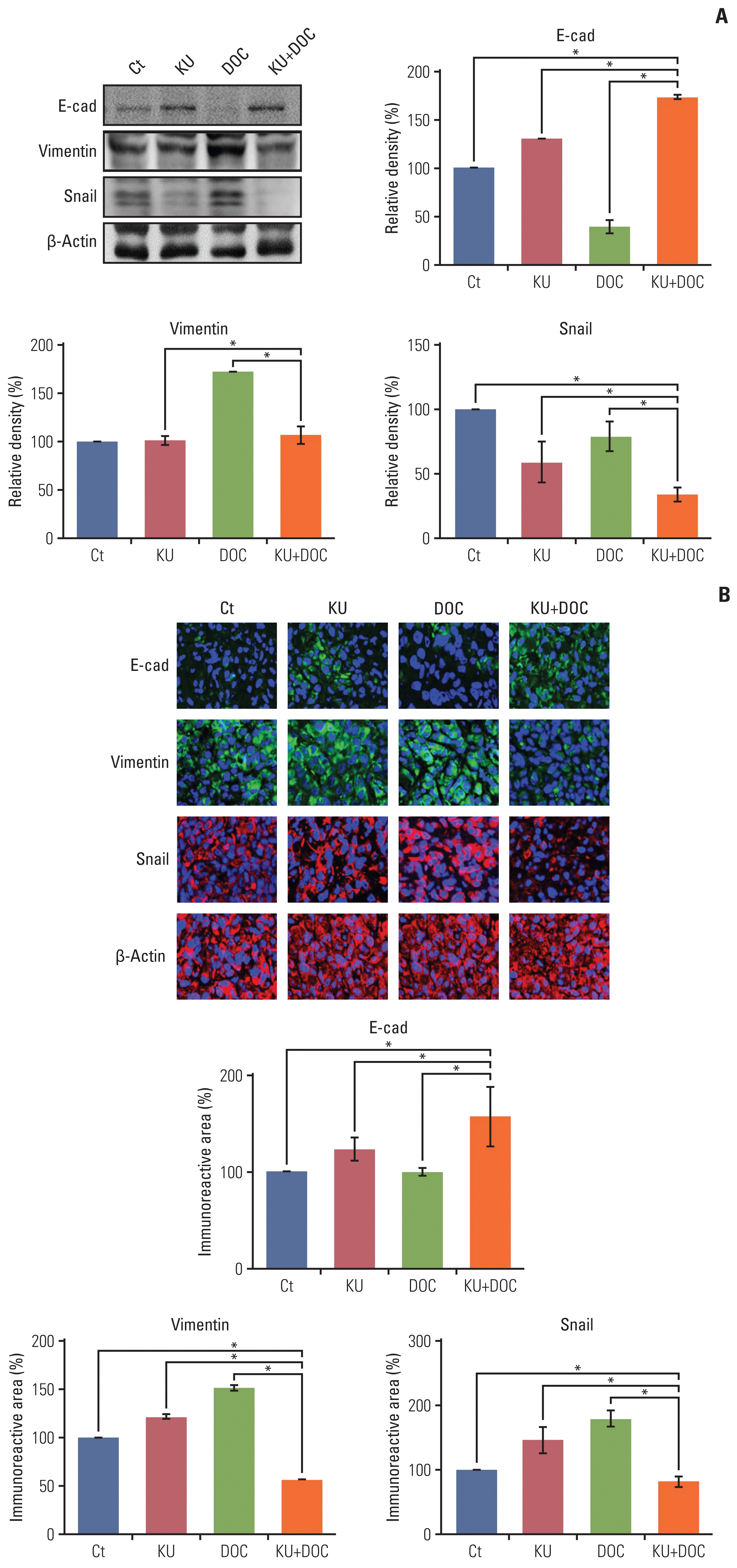 | Fig. 7Effects of each treatment on epithelial-mesenchymal transition (EMT) of triple-negative breast cancer (TNBC) cells in vivo. (A) Western blot analysis showing the expression of EMT-related markers in MDA-MB-231 TNBC cells xenografted in nude mice following docetaxel (DOC) and Ku-0063794 (KU) mono- and combination therapies. Relative densities of EMT-related markers. The relative densities had been quantified using Image J software and then were normalized to the density of β-actin in each group. (B) E-cadherin, snail, and vimentin immunofluorescence of MDA-MB-231 TNBC cells xenografted in nude mice following DOC and KU mono- and combination therapies. Percentages of immunoreactive areas were measured using NIH image J and expressed as relative values to the control. E-cad, E-cadherin; LC3B, microtubule-associated proteins 1A/1B light chain 3B. Values are presented as mean±standard deviation of three independent experiments. *p < 0.05. |




 PDF
PDF Citation
Citation Print
Print


 XML Download
XML Download