Abstract
Background:
Posterior reversible encephalopathy syndrome (PRES) is a neuroradiological syndrome characterized by headache, altered mental status, visual disturbance, and seizures with diagnostic MRI features, especially in the territories of posterior circulation. Reversibility of clinical and radiologic findings is generally regarded as a defining feature of PRES.
Case Report:
A 72-year old man who had a history of hypertension presented with subacute and progressive visual disturbance, dizziness, limb ataxia, and finally non-convulsive status epilepticus. Magnetic resonance imaging (MRI) showed extensive lesions in bilateral parieto-occipital cortex and subcortex. Due to his marked fluctuation of blood pressure, we detected a pheochromocytoma of left adrenal gland. In spite of administration with several types of antihypertensive medication, the patient presented with clinical deterioration, leading to death. MRI demonstrated the progression of lesions.
Posterior reversible encephalopathy syndrome (PRES) is a neuroradiological entity characterized by headache, vomiting, visual changes, seizures, altered mentality, and various neurologic symptoms. The clinical features are associated with potentially reversible cerebral edema, primarily in the territories of brain supplied by posterior circulation. The symptoms usually develop over a few hours and progression over several days is uncommon [1,2]. We report a patient with posterior encephalopathy syndrome who gradually deteriorated and died six months later.
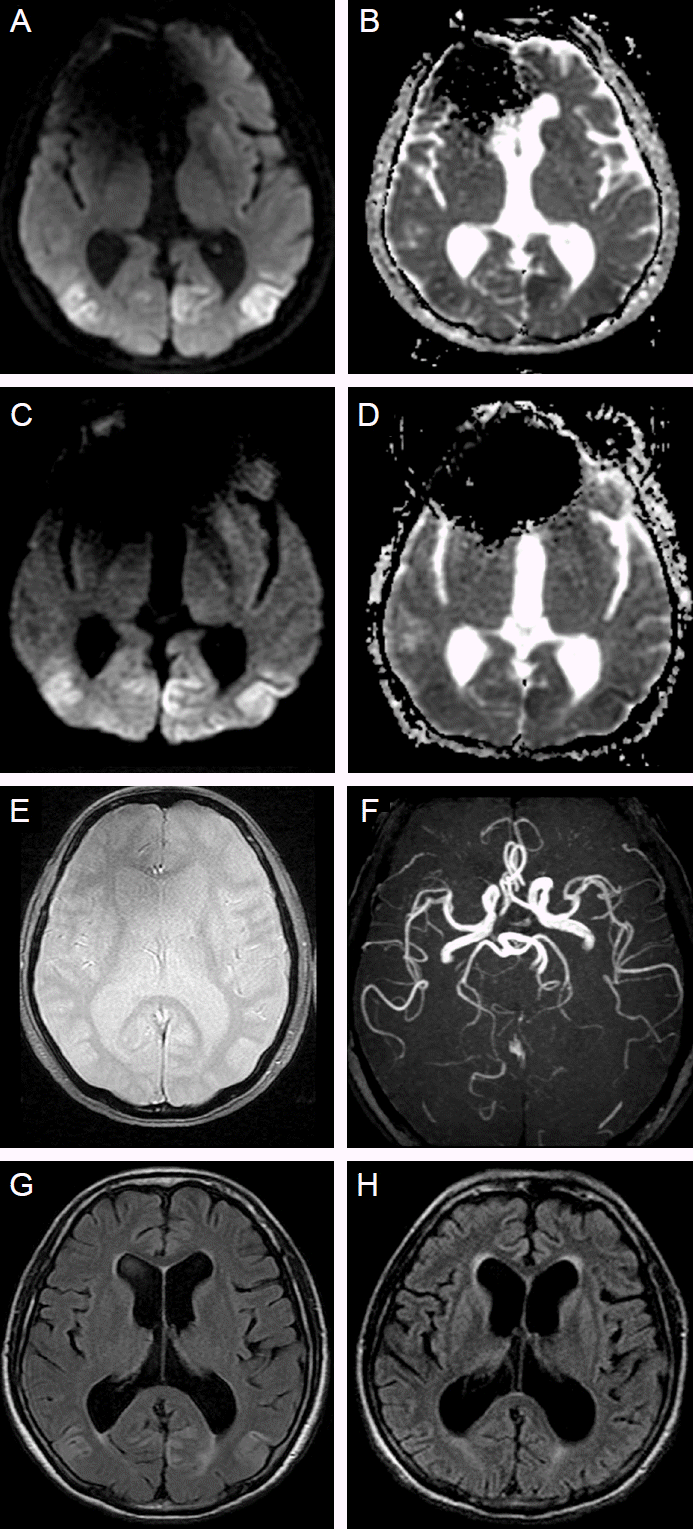
A 72-year-old man with a medical history of hypertension and angina pectoris had experienced dizziness and visual disturbance for 3 months. One month previous to presentation, the patient subacutely developed dysarthria and limb ataxia. Diffusion weighted image (DWI) and apparent diffusion coefficient (ADC) showed an extensive diffusion restriction signals in the bilateral parietooccipital area (Fig. 1A, B). First impression was multifocal infarction in the both posterior cerebral artery territories. However, the patient’s condition gradually deteriorated despite antiplatelet treatment at a secondary hospital. During a few days before presentation, underlying symptoms such as dizziness, visual disturbance, and dysarthria were progressed and behavioral change has occurred. Even then his electroencephalogram (EEG) showed diffuse slow activities in both hemispheres.
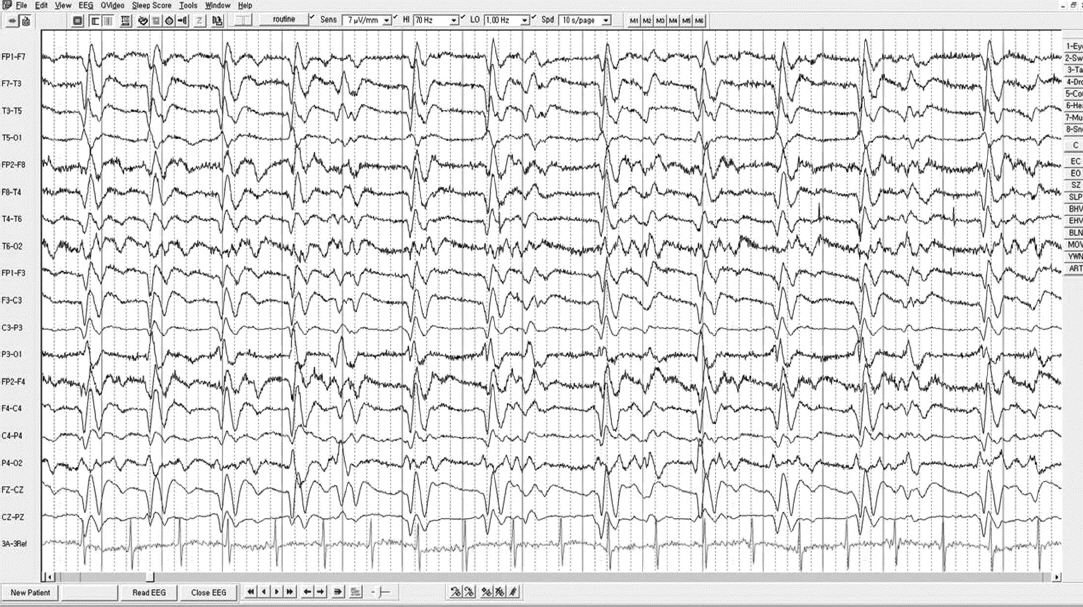
The patient became confused one day prior to presentation to our hospital. We performed second MRI, and which showed increased lesion size compared to previous imaging taken before one month (Fig. 1A-D). There were no punctuate hematomas indicative of amyloid encephalopathy in gradient echo image and magnetic resonance angiography in posterior circulation was normal (Fig. 1E, F). Underlying hypertension and radiologic findings supported a diagnosis of PRES. Second EEG showed generalized periodic epileptiform discharges (Fig. 2) and he was confused without recovery, so his clinical diagnosis was nonconvulsive status epilepticus (NCSE) . Administration of phenytoin, sodium valproate, oxcarbazepine, and levetriacetam was ineffective. Following the continuous infusion of bolus midazolam, EEG showed no more epileptiform discharges, but diffuse continuous slowing was still remained.
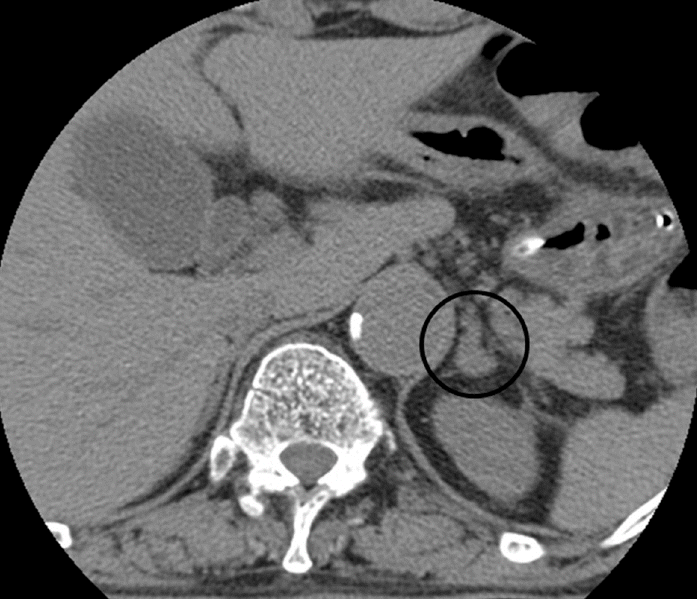
The patient’s blood pressure (BP) was 149/78 mmHg on admission. Thereafter, the systolic BP fluctuated widely from 98-184 mmHg, despite an administration of several types of antihypertensive medication (maximum doses of telmisartan, amlodipine, hydrochlorothiazide, diltiazem, carvedilol, nimodipine). To specify the reason of uncontrolled hypertension, we measured the level of hormones that could affect BP. Levels of urinary catecholamines included 2.4 mg/day metanephrine (normal range, 0.2-1.2 mg/day), 8.5 mg/day vanillylmandelic acid (normal range, 1-5 mg/day), 143.1 μg/day norepinephrine (normal range, 15-80 μg/day), and 59.10 μg/day epinephrine (normal range, 1.2-20 μg/day). The plasma catecholamine levels were also markedly increased (norepinephrine, 1.27 ng/mL [normal range, 0.07-0.4 ng/mL] and epinephrine, 0.45 ng/mL [normal range, 0.04-0.2 ng/mL]). Plasma levels of renin, aldosterone, and cortisol were within the normal ranges. Because these biochemical profiles were highly suggestive of pheochromocytoma, we performed an adrenal computed tomography and it showed a 9 x 10 mm mass in the left adrenal gland (Fig. 3).
The surgical removal of the adrenal mass was not tried due to the patient’s medical condition and family member’s opinion. Two months later, the follow-up brain MRI revealed diffuse cortical atrophy in fluid attenuated inversion recovery (FLAIR) image which was not remarkable in the previous image (Fig. 1G, H). The patient continued to be unresponsive and died after developing sepsis caused by pneumonia 3 months after admission.
The pathogenesis of PRES is still unclear. Two opposing theories have been proposed. The current popular hypothesis of the pathophysiology is that hypertension overcomes the upper limit of cerebral autoregulation, leading to vasodilation and vasogenic edema [3]. However, in contrast to typical MRI of PRES which shows predominant vasogenic edema, hyperintense signals on DWI and restricted diffusion on apparent diffusion coefficient (ADC) mapping, which are hallmarks of cytotoxic edema, were frequently observed in PRES [4,5]. In our patient, DWI and ADC findings showed cytotoxic edema. This change can be induced by PRES itself, but long-lasting BP surge, undetected seizure, or endothelial dysfunction due to circulating toxins can influence on it [2].
It was hard to make the conclusion about PRES rather than the possibility of unresponsive NCSE. The symptoms of the patient such as dysarthria and gait ataxia had slowly progressed from a month before admission, but he had not shown mental changes or seizure-like activities at that time. Based upon the clinical and radiologic findings, the first impression of previous hospital was multifocal stroke in posterior circulation. However, dizziness, visual disturbance, and dysarthria were deteriorated when he was admitted to our hospital, and the extent of lesion in DWI and FLAIR of the second MRI were increased only in the posterior circulation. Even then the EEG only showed diffuse slow activities in both hemispheres. We confirmed the diagnosis as PRES. After confused mentality has occurred, the second EEG showed changes compatible with NCSE.
Reversibility is generally regarded as a defining feature of PRES. However, it is not always so [1,2,5]. In our case, the extent of lesion increased in the interval between the DWI examinations. Several hypotheses were suggested to explain this finding. First, the clinical deterioration of the patient may have been directly influenced by a longlasting seizure. The parenchymal abnormalities evident in MRI may be a result, and not a cause, of seizure [1,2]. Second, systemic infection and sepsis can also influence the aggravation, since in a septic condition inflammatory cytokine release leads to endothelial dysfunction with subsequent vasoconstriction [2,4]. Third, a pheochromocytoma-induced hypertensive crisis can precipitate cerebrovascular manifestations. Sympathetic hormones secreted from phechromocytoma may mediate cerebral arterial constriction and vasospasm [6].
Pheochromocytoma is a neuroendocrine tumor that commonly arises from the adrenal gland. Because of the excessive secretion of catecholamines, pheochromocytoma can cause life-threatening hypertension. It is potentially curable if diagnosed and treated in time.
We report this case to highlight the clinical and radiological irreversibility of pheochrocytoma-induced posterior encephalopathy syndrome. Although reversibility is typical characteristics of PRES, a specific etiology such as pheochromocytoma and secondary complication including NCSE or sepsis can induce irreversibility.
REFERENCES
1. Lee VH, Wijdicks EF, Manno EM, Rabinstein AA. Clinical spectrum of reversible posterior leukoencephalopathy syndrome. Arch Neurol. 2008; 65:205–10.

2. Roth C, Ferbert A. The posterior reversible encephalopathy syndrome: what’s certain, what’s new? Pract Neurol. 2011; 11:136–44.

4. Bartynski WS. Posterior reversible encephalopathy syndrome, part 2: controversies surrounding pathophysiology of vasogenic edema. AJNR Am J Neuroradiol. 2008; 29:1043–9.

5. Covarrubias DJ, Luetmer PH, Campeau NG. Posterior reversible encephalopathy syndrome: prognostic utility of quantitative diffusion-weighted MR images. AJNR Am J Neuroradiol. 2002; 23:1038–48.
Figure 1.
Serial magnetic resonance images.Magnetic resonance imaging shows hyperintensity on DWI (A) and restricted diffusion on ADC (B) in bilateral parietooccipital lobes one month before admission. The abnormalities were progressed at admission (C, D). Gradient echo image and MR angiography did not show any abnormalities (E, F). Cortical atrophy progressed in the comparison between FLAIR image one month before admission (G) and that two months after admission (H).




 PDF
PDF Citation
Citation Print
Print


 XML Download
XML Download