Abstract
Purpose
The incidence of human papillomavirus (HPV)-related oropharyngeal cancer (OPC) has increased, and staging and optimal therapeutic approaches are challenging. A questionnaire survey was conducted to investigate the controversial treatment policy of stage T2 OPC according to the N category and determine the opinions of multidisciplinary experts in Korea.
Materials and Methods
Five OPC scenarios were developed by the Subcommittee on Oropharyngeal Treatment Guidelines of the Korean Society for Head and Neck Oncology and distributed to experts of multidisciplinary treatment hospitals.
Results
Sixty-five experts from 45 institutions responded. For the HPV-positive T2N0M0 scenario, 67.7% of respondents selected surgery followed by definitive concurrent chemoradiotherapy (CCRT) or radiotherapy alone. For the T2N1M0 HPV-positive scenario, there was a notable difference in the selection of primary treatment by expert specialty; 53.9% of respondents selected surgery and 39.8% selected definitive CCRT as the primary treatment. For the T2N3M0 advanced HPV-positive scenario, 50.0% of respondents selected CCRT and 33.3% considered induction chemotherapy (IC) as the primary treatment. CCRT and IC were significantly more frequently selected for the HPV-related OPC cases (p=0.010). The interdepartmental variability showed that the head and neck surgeons and medical oncologists favored surgery, whereas the radiation oncologists preferably selected definitive CCRT (p < 0.001).
Conclusion
In this study, surgery was preferred for lymph node-negative OPC, and as lymph node metastasis progressed, CCRT tended to be preferred, and IC was administered. Clinical practice patterns by stage and HPV status showed differences according to expert specialty. Multidisciplinary consensus guidelines will be essential in the future.
The incidence of oropharyngeal cancer (OPC) associated with human papillomavirus (HPV) has increased around the world and has recently been staged separately [1]. Data released in 2010 by the Centers for Disease Control and Prevention showed that the incidence of HPV-positive tonsil cancer is growing faster than that in the 1990s [2]. The incidence of HPV-positive (HPV(+)) is on the rise according to data published in the United States and the United Kingdom [3,4]. Many studies have been conducted on prognosis and treatment and have shown significantly better outcomes in these patients than in HPV-negative (HPV(−)) patients [5–7]. There are no statistical data confirming the incidence of HPV(+) OPC in the Republic of Korea, but the disease has a relatively higher overall incidence than that in other East Asian countries [8].
To achieve an increased cure rate and lower complication rate with these good prognostic diseases, proper multidisciplinary treatment guidelines are mandatory [9,10]. However, currently, there is heterogeneity among treatments and no appropriate consensus guidelines for multidisciplinary treatment according to HPV status [10]. Considering the high accessibility of multidisciplinary treatment in the Korean medical environment, appropriate evidence-based clinical guidelines for OPC according to HPV status are needed.
The purpose of this study was to investigate the current nationwide treatment policies for OPC and suggest opinions for preparing multidisciplinary consensus guidelines that are evidence-based and appropriately fit to the Korean medical environment. Since the amount is vast to investigate the treatment policy for entire OPC, this study focused on finding out the initial treatment decision for OPC, which may have disagreements among interdisciplinary. Therefore, the scenario was limited to T2 category and treatment policy according to the N category was reflected.
The survey was developed by the Subcommittee on Oropharyngeal Treatment Guidelines of the Korean Society for Head and Neck Oncology (KSHNO). The questionnaires asked about treatment policies for five scenarios depending on the stage or status of HPV infection. All cases were staged according to the American Joint Committee on Cancer (AJCC) 8th edition for both clinical and pathologic staging. Questionnaires were sent to all board-certified expert members of KSHNO who practice multimodal treatment of head and neck cancer in Korea. The questionnaire was sent via e-mail twice from July to August 2019, and the results were collected and analyzed in October 2019. The images and clinical summaries for the five scenarios are shown in Fig. 1.
A 61-year-old male patient without underlying disease visited with a sore throat. His performance status was Eastern Cooperative Oncology Group performance status (ECOG PS) 1, and he had a 10 pack-per-year (PPY) smoking history. After imaging study and biopsy, he was diagnosed with HPV(+) squamous cell carcinoma (SCC) with clinical stage T2N0M0, and there was no base of tongue invasion. The choice of the first treatment method for this patient was investigated.
A 52-year-old female patient, ECOG PS 1, was a non-smoker without underlying disease admitted for a 3.3-cm-sized mass in the right tonsil and multiple metastatic lymph nodes on the right level II–III, and there was no tongue base invasion. The final diagnosis was HPV(+) SCC, and the clinical stage was T2N1M0. The questionnaire asked about the first treatment modality of choice.
A 49-year-old female non-smoker and ECOG PS 1 without underlying disease visited for a sore throat and a palpable huge mass on the left neck. A 4-cm-sized mass without base of tongue invasion in the left tonsil and metastatic lymph nodes in the ipsilateral upper, middle, and lower internal jugular chain were observed. A biopsy confirmed HPV(+) SCC, and the clinical stage was T2N3M0. The questionnaire asked about the first treatment for this patient.
A 69-year-old man, ECOG PS 1, with a 30-PPY smoking history visited the hospital with a sore throat. On magnetic resonance imaging, a mass of 2.2 cm, with the longest diameter in the left tonsil, was suspicious of tongue base invasion, and a 1.7-cm-sized lymph node metastasis was seen at ipsilateral cervical level II. He was diagnosed with HPV(−) SCC, and the clinical stage was T2N1M0. The first treatment for his cancer was queried.
A 59-year-old male 20-PPY smoker visited the hospital for a sore throat. His pretreatment performance status was ECOG PS 1. HPV(−) SCC was diagnosed as clinical stage T2N2bM0 with a 2-cm-sized mass without tongue base invasion in the right tonsil and several lymph node metastases at ipsilateral cervical level II–III. The first treatment for his cancer was queried.
The questionnaires were sent to experts in treating head and neck tumors from 86 institutions in Korea with more than 300 beds, and responses were received from individuals or multidisciplinary teams representing the institutions. Multidisciplinary team gathered together in each department to derive the consensus opinion and submit the answer. The content of the returned data was anonymized and analyzed. In the questionnaire, the professional medical department, the period elapsed since board certification, the status of multidisciplinary consultation, and the method of surgery and radiation therapy in the institutions were surveyed. A chi-square test was performed to evaluate the relationship between treatment choice and each factor, such as HPV status, number of nodal metastases, department of experts, hospital beds, robotic surgery availability, and consultation meeting, and a p-value of less than 0.05 was considered statistically significant. Statistical analyses were performed using SPSS ver. 24 (IBM Corp., Armonk, NY).
A total of 59 individuals and six multidisciplinary teams from 45 institutions responded. The nationwide response rate was 52.3% (45/86 institutions). Thirteen out of 16 institutions with > 1,000 hospital beds (81.3%), 29 out of 60 institutions with 500–1,000 hospital beds (48.3%), and three out of 10 institutions with > 300 and ≤ 500 hospital beds (30%) replied to the survey, indicating a high response rate in institutions with a high number of hospital beds. The most responses were from experts from the department of radiation oncology (n=35, 53.8%), followed by head and neck surgeons (n=16, 24.6%), medical oncologists (n=8, 12.3%), and multidisciplinary teams (n=6, 9.2%).
The institutional surveys reported that 69.2% decided treatment modalities through regular head and neck conferences, 10.8% held meetings if necessary, and 18.5% decided through interdepartmental patient referrals. Robotic surgery was available in 67.7% of the institutions, and most respondents (93.8%), except for one, performed radiotherapy for head and neck tumors using the intensity-modulated radiotherapy technique. Data on the characteristics of the respondents are summarized in Table 1.
A survey of the first treatment was conducted for both HPV(+) and HPV(−) OPC cases. The selection of the first treatment for each case is presented in Fig. 2. For the responses to HPV(+) case 1 (T2N0M0), 67.7% of the respondents selected surgery as the first treatment followed by definitive concurrent chemoradiotherapy (CCRT) or radiotherapy alone. In the responses to the first treatment for resectable case 2 (HPV(+) T2N1M0 with multiple cervical lymph node metastases in the ipsilateral region), surgery was favored by 53.9% and definitive CCRT by 39.8%. In the more advanced stage of case 3 (HPV(+) T2N3M0), CCRT was preferred by 50.0% of the respondents, followed by induction chemotherapy in 33.3% and surgery in 16.7%.
The results of the questionnaire for the primary treatment of HPV(−) OPC cases (cases 4 and 5) are shown in Fig. 2D and E. In the T2N1M0 HPV(−) OPC case (case 4), surgery was preferred by 64.5% of the respondents, and in case of multiple metastatic lymph nodes observed in the ipsilateral cervical lymph node (case 5, HPV(−) T2N2bM0), definitive CCRT was the most preferred, by 42.9% of the respondents.
For four scenarios, excluding case 1 without metastatic lymph nodes, a chi-square test was performed on the association between selection of treatment method and HPV status. CCRT and induction chemotherapy was more frequently chosen for the HPV(+) cases than for the HPV(−) cases, and the difference was significant (p=0.010; odds ratio, 9.276) (Fig. 3).
The difference in the treatment options selected according to specialty was remarkable in each scenario. In Fig. 2, the treatment methods selected by the different expert departments by case are shown as a graph. Among them, for case 2 (HPV(+) T2N1M0 with multiple cervical lymph node metastases in the ipsilateral region), inter-department variability clearly appeared. The head and neck surgeons and medical oncologists favored surgery (63.3% and 68.8%), whereas 54.3% of the radiation oncologists selected definitive CCRT (Fig. 2B). The difference in treatment policy between departments was statistically significant, with an odds ratio of 30.911 and a p-value less than 0.001. Table 2 summarizes the results of the treatment modalities and factors related to the scenarios and respondents.
A significant difference was found according to the stage of the metastatic lymph node (Fig. 4). Compared to that for the N1 stage, CCRT and induction chemotherapy were more frequently selected for advanced lymph node N2 or N3 categories. This difference was statistically significant (odds ratio, 31.437; p < 0.001).
The number of hospital beds, the availability of robotic surgery, and the status of the consultation meeting were analyzed to determine trends by scenario (Table 2). Graphs for the proportion of responses according to these factors are shown in S1 Fig. Number of hospital beds showed a difference in practice patterns. In institutions with a higher number of beds, a high proportion of respondents from institutions with more than 500 beds selected CCRT for both HPV(+) and HPV(−) cases compared to those from institutions with less than 500 beds. Additionally, in case 3 (HPV(+) T2N3M0 with ipsilateral bulky upper and lower neck node metastases), a more advanced stage, approximately 50% of respondents from institutions with ≤ 500 beds selected induction chemotherapy; however, respondents from institutions with > 500 beds more frequently selected CCRT (Fig. 5). Nevertheless, these factors did not show a statistically significant difference in treatment choice (> 1,000 vs. > 500 and ≤ 1,000 vs. > 300 and ≤ 500, p=0.149). When analyzed based on 500 beds, there were marginally significant differences (> 500 vs. ≤ 500, p=0.067). There was no significant difference in the availability of robotic surgery and the status of the consultation meeting according to treatment options (p=0.280 and p=0.781, respectively).
This study is meaningful as the first multidisciplinary survey study of OPC in Korea. The purpose of this study was to survey the current treatment patterns of OPC which may have disagreements interdisciplinary and suggest the need for evidence-based multidisciplinary consensus guidelines suitable for the Korean medical environment. We investigated initial treatment selection limited to T2, according to N category where various treatment strategies can be selected.
The mainstay treatment for OPC includes surgery and definitive chemoradiation or radiation alone. Recently, the de-intensification of treatment according to HPV status was proposed [11]. However, the proposal was based on retrospective studies, and the study periods were generally short. Thus, the evidence is insufficiently powered to change the treatment paradigm [12,13]. The current OPC treatment guidelines are not quite different for HPV-negative and HPV-positive patients, even suggested treatment according to risk stratification in HPV(+) disease. The initial stages of T1-2 and N0-1 OPC are treated with resection of the primary tumor and neck dissection or definitive radiotherapy. The French Head and Neck Cancer Group (GORTEC) trial and a retrospective study of the National Cancer Database (NCDB) reported that definitive CCRT could be used as a treatment option in P16-negative N1 disease, and for advanced-stage OPC, curative CCRT was recommended because of the high morbidity risk due to trimodality treatment including adjuvant CCRT after surgery [14,15]. In particular, for T4 or N3 disease, definitive CCRT is definitely preferred. Therefore, treatments for HPV(+) OPC of stage T2N0-2 and HPV(−) OPC of stage T2N1-2, where options for both surgery and definitive CCRT are available, vary widely between individuals and institutions. In addition, induction chemotherapy has been provided for indications based on RTOG 9111 in hypopharyngeal or laryngeal cancer as organ preservation aim, but the consensus in OPC is still insufficient to improve treatment outcomes [16]. This diversity in treatment guidelines has become a factor increasing the gap between experienced experts and institutions, making it difficult to improve the quality of treatment for OPC.
The results of this study showed differences in treatment policy according to HPV status and extent of nodal stage. Clinical studies on the treatment method for HPV(+) OPC are currently being actively conducted. In definitive CCRT, cetuximab was inferior in overall survival and progression-free survival (PFS) and had increased locoregional and distant failure rates compared to cisplatin in the phase III randomized clinical studies RTOG 1016 and De-ESCALaTE, in which cetuximab was substituted for cisplatin [17,18]. In the NCT01530997 phase 2 study, patients with T0-3N0-2c HPV(+) OPC with less than 10 PPY of smoking history were studied and the radiation dose and the dose of chemotherapeutic regimens in definitive CCRT were lowered; 86% showed pathologic complete response and relatively comparable locoregional control and overall survival [19]. In the phase 2 study NRG-HN002, CCRT and radiotherapy alone (60 Gy radiotherapy) were compared in T1-2N1-N2bM0 or T3N0-N2bM0 HPV(+) OPC patients with ≤ 10 PPY of smoking history, and the 2-year PFS was 90.5% in the CCRT group and 87.6% in the radiotherapy alone group [20]. Other phase 2 and 3 studies (NCT01855451, NCT03077243, NCT04106362, and NCT03822897) on the de-intensification of definitive CCRT are currently in progress.
The preferred treatment method for each specialty showed different trends. Head and neck surgeons responded that advanced surgical techniques could be used and various surgical instruments have been developed, so surgical treatment was possible in OPC even at advanced stages [21]. However, radiation oncologists favored definitive CCRT, noting that it could achieve good tumor control while preserving organ function. These perspectives were also revealed by differences between departments in surveys of treatment methods for other cancers [22]. This debate is inevitable because each treatment has its pros and cons and will continue until the results of a large-scale clinical study show whether one treatment is definitively superior in terms of the long-term outcomes and toxicities. In our survey, depending on whether or not a consultative meeting was held, the treatment policy could be different. Therefore, the treatment policy should be determined through more multidisciplinary meetings in the future. In addition, it is necessary to establish a consensus among societies to provide optimal therapy and reduce differences in each institution. A detailed questionnaire on radiation therapy was additionally conducted, and its analysis will be published in another paper in the near future.
The reason why the treatment choice is different is due to concerns about complications. Definitive CCRT for OPC is associated with increased toxicity, such as long-term dysphagia. A gastrostomy insertion rate of 24% at one year and 14% at 2 years after CCRT has been reported, and xerostomia is one of the leading causes associated with radiation-related complications [23]. On the other hand, the reduction of swallowing function and fear of the sacrifice of functional and aesthetic organs from surgery are the reasons for choosing induction chemotherapy or radiation therapy [24]. The reason why there are so many debates in each specialty may be due to the different viewpoints of concern about these treatment-related complications.
The interesting point of this study is that induction chemotherapy was chosen as the first treatment in the cT2N3M0 HPV(+), even in the National Comprehensive Cancer Network (NCCN) guideline category 3 [25]. Nevertheless, it was analyzed that about half of surgeons and medical oncologists preferred induction chemotherapy in OPC with relatively high lymph node stage. Expected role of induction chemotherapy was for reduction of distant metastases as causes of treatment failure. From previous studies, the role of induction chemotherapy in the treatment of locally advanced OPC is still highly debated [26–28]. Taxane-based induction chemotherapy showed superior effects over non-taxane-based combinations in a randomized phase III trial [29,30]. However, induction chemotherapy followed by CCRT or surgery did not show any clinical advantage compared to CCRT or sequential surgery followed by CCRT. Therefore, induction chemotherapy should not be regularly treated in OPC patients. However, if the multidisciplinary team decided the case was difficult to perform surgery or chemoradiotherapy initially, such as bulky matted N3 disease or extensive primary tumor with adjacent structure invasion, induction chemotherapy followed by surgery or chemoradiotherapy might be a careful treatment option. And also, the potential role of induction chemotherapy as de-escalation regimen in HPV(+) OPC would be defined in the future.
There were several limitations to this survey study. First, there were insufficient questions on surgical techniques for OPC. Although the questions on treatment method selection were surveyed by stage, opinions on surgical techniques were not objectively surveyed, as the differences were likely to vary depending on the surgical environment and equipment in the institution or the head and neck surgeon’s experience and perspectives. In addition, the treatment policy according to the N category was reflected, but questions according to the T category were insufficient. This is because, in general, surgery is recommended for T1, and induction chemotherapy or definitive CCRT is preferred for T3–4. Thus, the approaches are fairly consistent, so the scenario was limited to T2. However, there will be limitations in establishing an algorithm for the overall treatment of OPC.
The results of the expert questionnaire study reflected that the debate on the choice of treatment method was based on the perspective of the professional field and showed that this could be an obstacle to the preparation of clinical evidence. The 2019 American Society of Clinical Oncology and 2017 American Society for Radiation Oncology guidelines for OPC did not address the consensus of primary treatment [9,10]. The NCCN guidelines also recommend various options as primary treatments for each stage, so there are no definite guidelines for each treatment method [25]. The prospects for the omission and de-escalation of adjuvant treatment in HPV(+) OPC patients will be discussed in the near future. In HPV(+) OPC, a good prognosis and a better response to radiation and chemotherapy may lead to changes in treatment options. We propose to plan a prospective study on the treatment strategies of OPC in the future, comparing treatment outcomes based on HPV status and stage.
In summary, the current survey of the clinical practice patterns of OPC in Korea showed that surgery was preferred for lymph node-negative OPC, and as lymph node metastasis progressed, CCRT tended to be preferred, and induction chemotherapy was also applied. A treatment consensus among multidisciplinary departments through active communication in academic societies like the KSHNO is needed to provide optimal therapy and reduce differences at each institution.
We observed different treatment policies between HPV-positive and HPV-negative patients in this survey. In addition, this survey will serve as the basis for creating a unified treatment guideline that takes into account differences in each hospital size or among experts. This will be the basis for treating OPC, considering both tumor control and complications, and establishing a treatment protocol that can be delivered at a reasonable social cost.
Electronic Supplementary Material
Supplementary materials are available at Cancer Research and Treatment website (https://www.e-crt.org).
Notes
Author Contributions
Conceived and designed the analysis: Choi KH, Song JH, Kim YS.
Collected the data: Choi KH, Kim YS, Kim JH, Jeong WJ, Nam IC, Kim JH, Ahn HK, Chun SH, Hong HJ, Joo YH, Eun YG, Moon SH, Lee J.
Contributed data or analysis tools: Choi KH, Song JH, Kim YS, Kim JH, Jeong WJ, Nam IC, Kim JH, Ahn HK, Chun SH, Hong HJ, Joo YH, Eun YG, Moon SH, Lee J.
Performed the analysis: Choi KH, Song JH, Kim YS, Kim JH, Jeong WJ, Nam IC, Kim JH, Ahn HK, Chun SH, Hong HJ, Joo YH, Eun YG, Moon SH, Lee J.
Wrote the paper: Choi KH, Kim YS.
ACKNOWLEDGMENTS
This work was supported by the Subcommittee on Oropharyngeal Treatment Guidelines of the KSHNO. We thank all of KSHNO respondents to this survey and experts treating head and neck tumors from 45 institutions who participated in this survey as follows: Asan Medical Center, Yonsei University Severance Hospital, Samsung Medical Center, Seoul National University Hospital, Gachon University Gil Medical Center, The Catholic University of Korea Seoul St. Mary’s Hospital, Chungnam National University Hospital, Bundang Seoul National University Hospital, Pusan National University Hospital, Ajou University Hospital, Jeonbuk National University Hospital, Korea University Anam Hospital, Korea University Guro Hospital, Kosin University Gopspel Hospital, Dong-A University Hospital, Keimyung University Dongsan Medical Center, Inha University Hospital, Soonchunhyang University Cheonan Hospital, Gyeongsang National University Hospital, Daegu Catholic University Medical Center, Ewha Womans University Medical Center, Hanyang University Seoul Hospital, Wonju Severance Christian Hospital, Chosun University Hospital, Kyung Hee University Hospital, The Catholic University of Korea Incheon St. Mary’s Hospital, Yonsei University Gangnam Severance Hospital, Inje University Busan Paik Hospital, Chungbuk National University Hospital, The Catholic University of Korea St. Vincent’s Hospital, Wonkwang University Hospital, The Catholic University of Korea Uijeongbu St. Mary’s Hospital, Chonnam National University Hwasun Hospital, Kangbuk Samsung Hospital, The Catholic University of Korea Daejeon St. Mary’s Hospital, Kyung Hee University Hospital at Gangdong, The Catholic University of Korea Bucheon St. Mary’s Hospital, Presbyterian Medical Center, National Cancer Center, Catholic Kwandong University International St. Mary’s Hospital, Merinol Hospital, Korea Cancer Center Hospital, The Catholic University of Korea Yeouido St. Mary’s Hospital, Hanyang University Guri Hospital, and The Catholic University of Korea Eunpyeong St. Mary’s Hospital.
References
1. O’Sullivan B, Huang SH, Su J, Garden AS, Sturgis EM, Dahlstrom K, et al. Development and validation of a staging system for HPV-related oropharyngeal cancer by the International Collaboration on Oropharyngeal cancer Network for Staging (ICON-S): a multicentre cohort study. Lancet Oncol. 2016; 17:440–51.
2. Ramqvist T, Dalianis T. Oropharyngeal cancer epidemic and human papillomavirus. Emerg Infect Dis. 2010; 16:1671–7.

3. Chaturvedi AK, Engels EA, Pfeiffer RM, Hernandez BY, Xiao W, Kim E, et al. Human papillomavirus and rising oropharyngeal cancer incidence in the United States. J Clin Oncol. 2011; 29:4294–301.

4. Schache AG, Powell NG, Cuschieri KS, Robinson M, Leary S, Mehanna H, et al. HPV-related oropharynx cancer in the United Kingdom: an evolution in the understanding of disease etiology. Cancer Res. 2016; 76:6598–606.

5. Elrefaey S, Massaro MA, Chiocca S, Chiesa F, Ansarin M. HPV in oropharyngeal cancer: the basics to know in clinical practice. Acta Otorhinolaryngol Ital. 2014; 34:299–309.
6. Urban D, Corry J, Rischin D. What is the best treatment for patients with human papillomavirus-positive and -negative oropharyngeal cancer? Cancer. 2014; 120:1462–70.

7. Lee S, Lee SW, Park S, Yoon SM, Park JH, Song SY, et al. Refining prognostic stratification of human papillomavirus-related oropharyngeal squamous cell carcinoma: different prognosis between T1 and T2. Radiat Oncol J. 2017; 35:233–40.

8. Kim YT, Serrano B, Lee JK, Lee H, Lee SW, Freeman C, et al. Burden of human papillomavirus (HPV)-related disease and potential impact of HPV vaccines in the Republic of Korea. Papillomavirus Res. 2019; 7:26–42.

9. Sher DJ, Adelstein DJ, Bajaj GK, Brizel DM, Cohen EE, Halthore A, et al. Radiation therapy for oropharyngeal squamous cell carcinoma: executive summary of an ASTRO Evidence-Based Clinical Practice Guideline. Pract Radiat Oncol. 2017; 7:246–53.

10. Koyfman SA, Ismaila N, Crook D, D’Cruz A, Rodriguez CP, Sher DJ, et al. Management of the neck in squamous cell carcinoma of the oral cavity and oropharynx: ASCO Clinical Practice Guideline. J Clin Oncol. 2019; 37:1753–74.

11. Psyrri A, Rampias T, Vermorken JB. The current and future impact of human papillomavirus on treatment of squamous cell carcinoma of the head and neck. Ann Oncol. 2014; 25:2101–15.

12. O’Sullivan B, Huang SH, Siu LL, Waldron J, Zhao H, Perez-Ordonez B, et al. Deintensification candidate subgroups in human papillomavirus-related oropharyngeal cancer according to minimal risk of distant metastasis. J Clin Oncol. 2013; 31:543–50.
13. Masterson L, Moualed D, Masood A, Dwivedi RC, Benson R, Sterling JC, et al. De-escalation treatment protocols for human papillomavirus-associated oropharyngeal squamous cell carcinoma. Cochrane Database Syst Rev. 2014; (2):CD010271.

14. Denis F, Garaud P, Bardet E, Alfonsi M, Sire C, Germain T, et al. Final results of the 94-01 French Head and Neck Oncology and Radiotherapy Group randomized trial comparing radiotherapy alone with concomitant radiochemotherapy in advanced-stage oropharynx carcinoma. J Clin Oncol. 2004; 22:69–76.

15. Zumsteg ZS, Kim S, David JM, Yoshida EJ, Tighiouart M, Shiao SL, et al. Impact of concomitant chemoradiation on survival for patients with T1-2N1 head and neck cancer. Cancer. 2017; 123:1555–65.

16. Forastiere AA, Zhang Q, Weber RS, Maor MH, Goepfert H, Pajak TF, et al. Long-term results of RTOG 91–11: a comparison of three nonsurgical treatment strategies to preserve the larynx in patients with locally advanced larynx cancer. J Clin Oncol. 2013; 31:845–52.

17. Gillison ML, Trotti AM, Harris J, Eisbruch A, Harari PM, Adelstein DJ, et al. Radiotherapy plus cetuximab or cisplatin in human papillomavirus-positive oropharyngeal cancer (NRG Oncology RTOG 1016): a randomised, multicentre, non-inferiority trial. Lancet. 2019; 393:40–50.
18. Mehanna H, Robinson M, Hartley A, Kong A, Foran B, Fulton-Lieuw T, et al. Radiotherapy plus cisplatin or cetuximab in low-risk human papillomavirus-positive oropharyngeal cancer (De-ESCALaTE HPV): an open-label randomised controlled phase 3 trial. Lancet. 2019; 393:51–60.
19. Chera BS, Amdur RJ, Tepper J, Qaqish B, Green R, Aumer SL, et al. Phase 2 trial of de-intensified chemoradiation therapy for favorable-risk human papillomavirus-associated oropharyngeal squamous cell carcinoma. Int J Radiat Oncol Biol Phys. 2015; 93:976–85.

20. Yom SS, Torres-Saavedra P, Caudell JJ, Waldron JN, Gillison ML, Truong MT, et al. NRG-HN002: a randomized phase II trial for patients with p16-positive, non-smoking-associated, locoregionally advanced oropharyngeal cancer. Int J Radiat Oncol Biol Phys. 2019; 105:684–5.

21. Golusinski W, Golusinska-Kardach E. Current role of surgery in the management of oropharyngeal cancer. Front Oncol. 2019; 9:388.

22. Palmer MJ, O’Sullivan B, Steele R, Mackillop WJ. Controversies in the management of non-small cell lung cancer: the results of an expert surrogate study. Radiother Oncol. 1990; 19:17–28.

23. Machtay M, Moughan J, Trotti A, Garden AS, Weber RS, Cooper JS, et al. Factors associated with severe late toxicity after concurrent chemoradiation for locally advanced head and neck cancer: an RTOG analysis. J Clin Oncol. 2008; 26:3582–9.

24. Kolokythas A. Long-term surgical complications in the oral cancer patient: a comprehensive review. Part I. J Oral Maxillofac Res. 2010; 1:e1.

25. NCCN guidelines for head and neck cancer [Internet]. Plymouth Meeting, PA: National Comprehensive Cancer Network;2020. [cited 2020 Sep 11]. Available from: https://www.nccn.org/professionals/physician_gls/pdf/head-and-neck.pdf
.
26. Cohen EE, Karrison TG, Kocherginsky M, Mueller J, Egan R, Huang CH, et al. Phase III randomized trial of induction chemotherapy in patients with N2 or N3 locally advanced head and neck cancer. J Clin Oncol. 2014; 32:2735–43.

27. Haddad R, O’Neill A, Rabinowits G, Tishler R, Khuri F, Adkins D, et al. Induction chemotherapy followed by concurrent chemoradiotherapy (sequential chemoradiotherapy) versus concurrent chemoradiotherapy alone in locally advanced head and neck cancer (PARADIGM): a randomised phase 3 trial. Lancet Oncol. 2013; 14:257–64.

28. Hitt R, Grau JJ, Lopez-Pousa A, Berrocal A, Garcia-Giron C, Irigoyen A, et al. A randomized phase III trial comparing induction chemotherapy followed by chemoradiotherapy versus chemoradiotherapy alone as treatment of unresectable head and neck cancer. Ann Oncol. 2014; 25:216–25.
29. Posner MR, Hershock DM, Blajman CR, Mickiewicz E, Winquist E, Gorbounova V, et al. Cisplatin and fluorouracil alone or with docetaxel in head and neck cancer. N Engl J Med. 2007; 357:1705–15.

30. Lorch JH, Goloubeva O, Haddad RI, Cullen K, Sarlis N, Tishler R, et al. Induction chemotherapy with cisplatin and fluorouracil alone or in combination with docetaxel in locally advanced squamous-cell cancer of the head and neck: long-term results of the TAX 324 randomised phase 3 trial. Lancet Oncol. 2011; 12:153–9.

Fig. 1
Maximum intensity projection images of PET and clinical stage for five scenarios. AJCC, American Joint Committee on Cancer; HPV(+), human papillomavirus positive; HPV(−), human papillomavirus negative; PET, positron-emission tomography; PPY, pack-per-year.
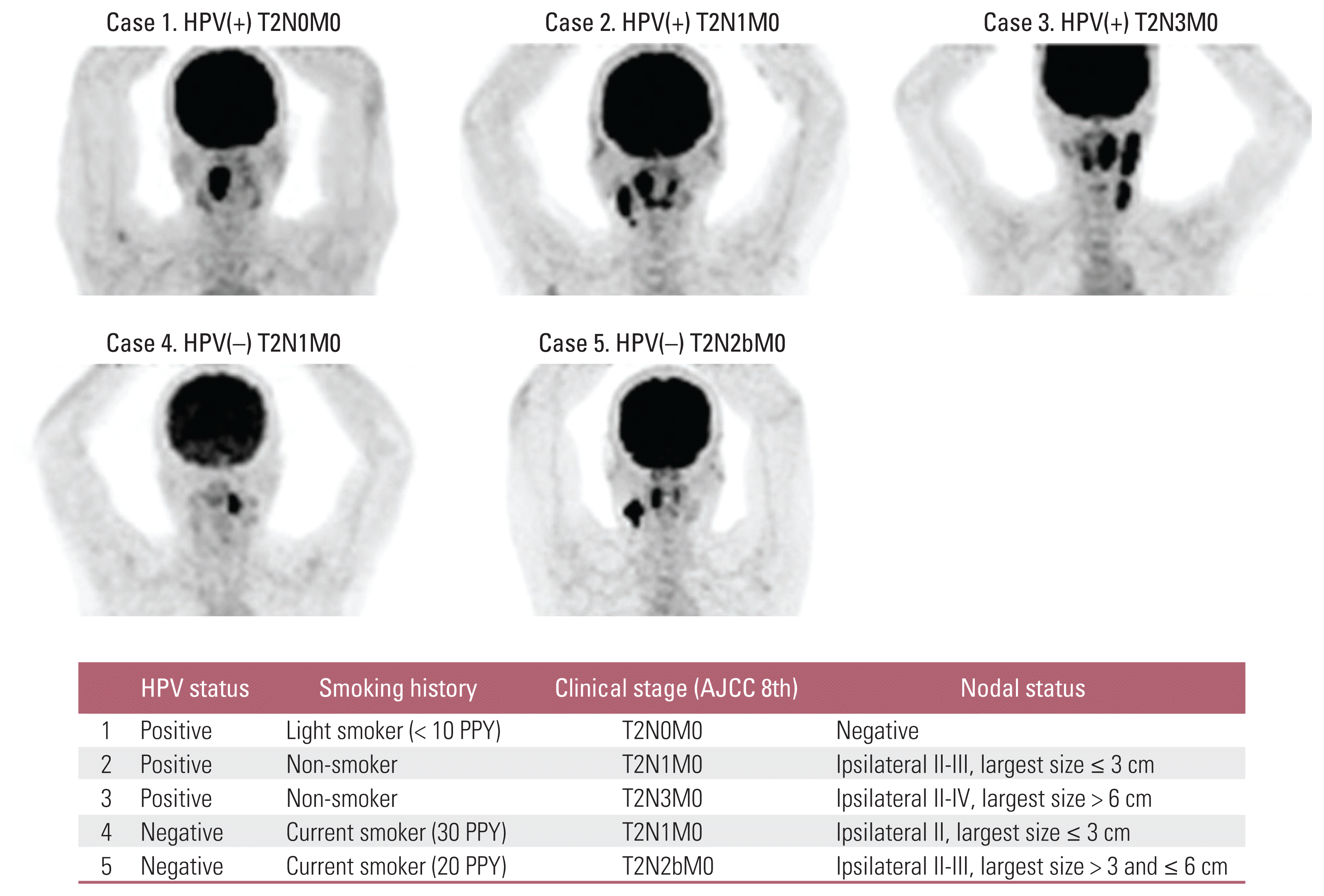
Fig. 2
Graphs for the proportion of responses according to the experts’ specialty in five scenarios (A–E, cases 1–5). The numbers in the stacked bar chart indicate each percentage of respondents who chose the treatment modality. CCRT, concurrent chemoradiotherapy; CTx, chemotherapy; HPV(+), human papillomavirus positive; HPV(−), human papillomavirus negative; RT, radiotherapy.
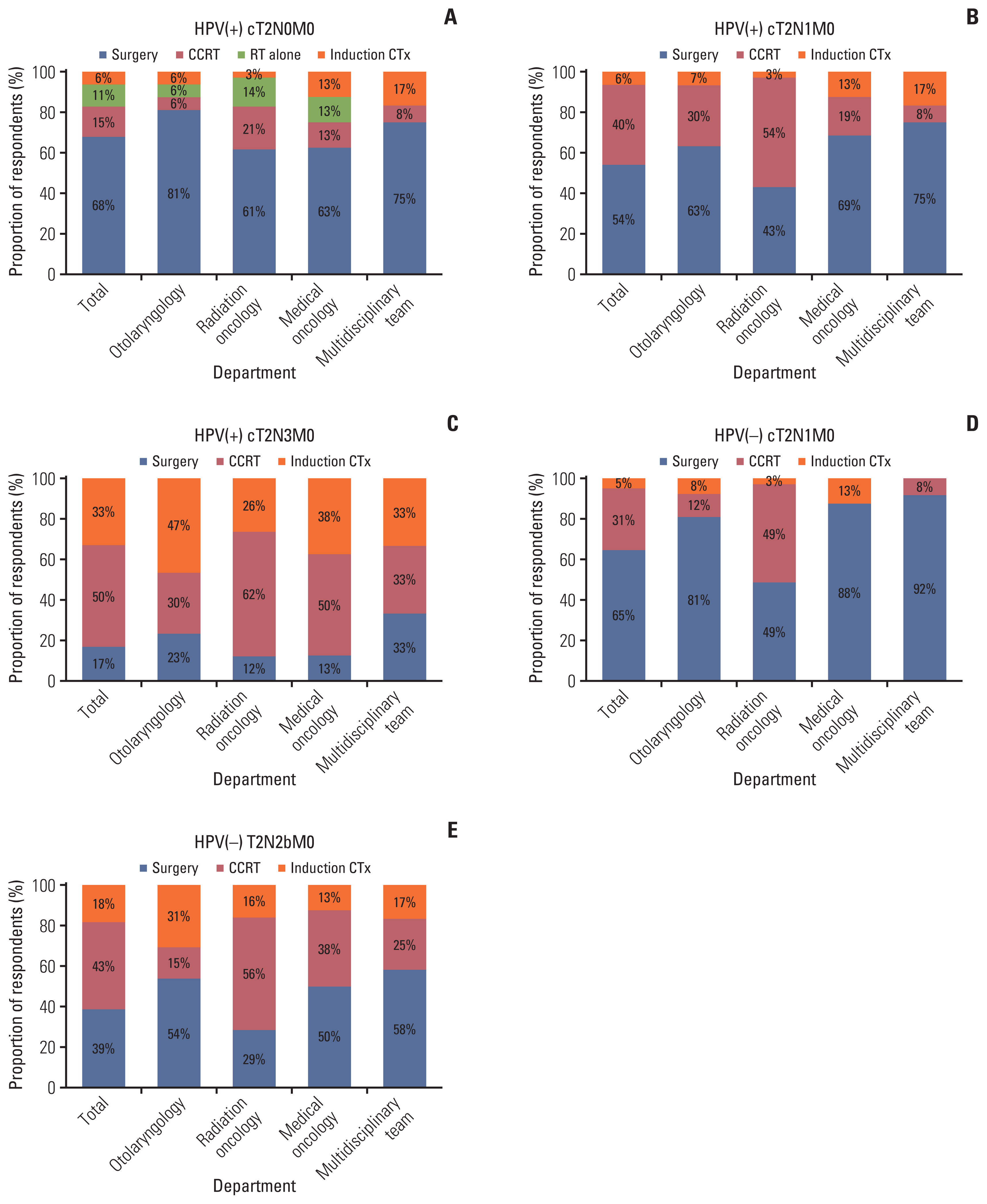
Fig. 3
Graphs for the proportion of responses according to HPV status. CCRT, concurrent chemoradiotherapy; CTx, chemotherapy; HPV, human papillomavirus.
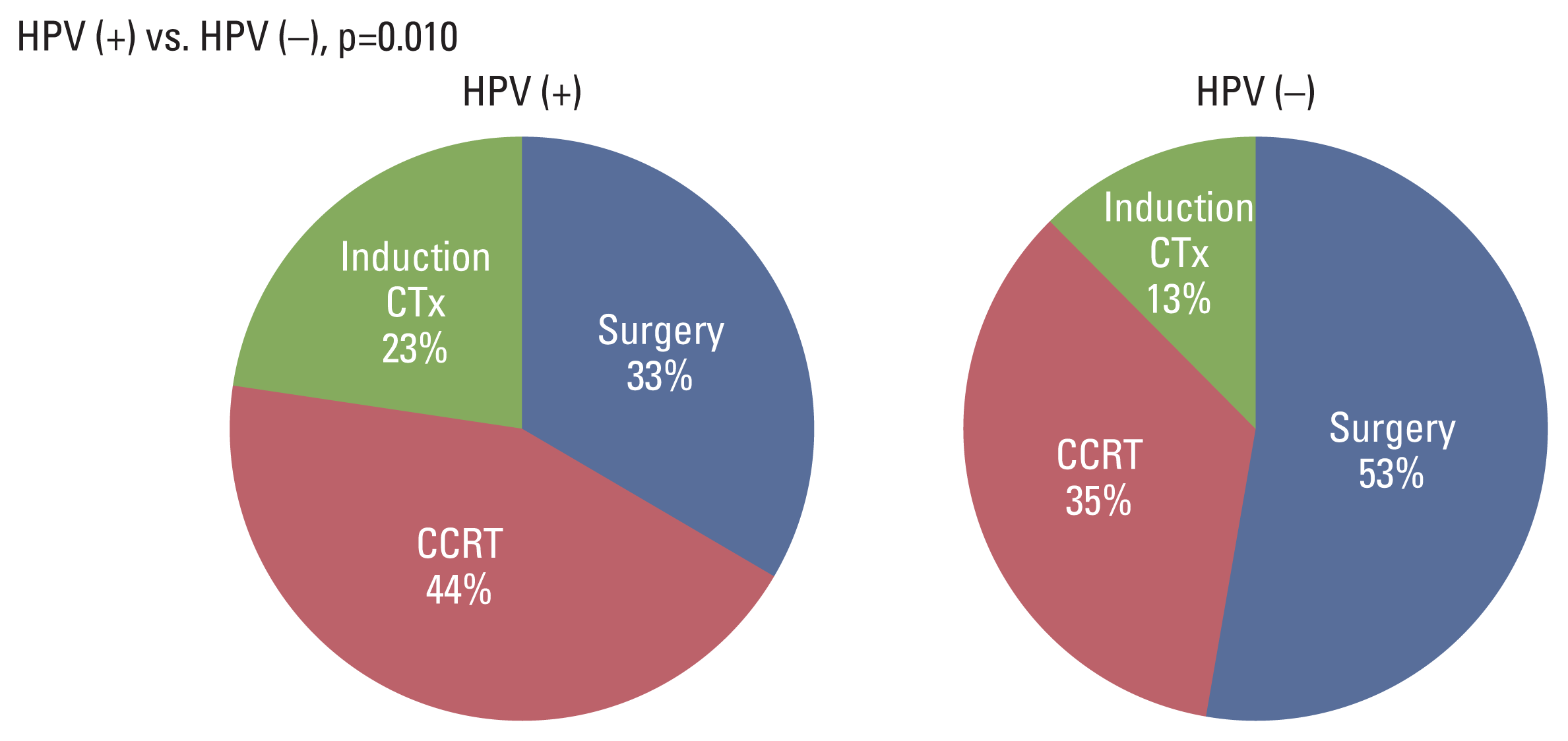
Fig. 4
Graphs for the proportion of responses according to nodal stage. CCRT, concurrent chemoradiotherapy; CTx, chemotherapy.
Fig. 5
Graphs for the proportion of responses according to hospital beds: T2N1M0 cases (A) and T2N2bM0 or T2N3M0 cases (B). CCRT, concurrent chemoradiotherapy; CTx, chemotherapy.
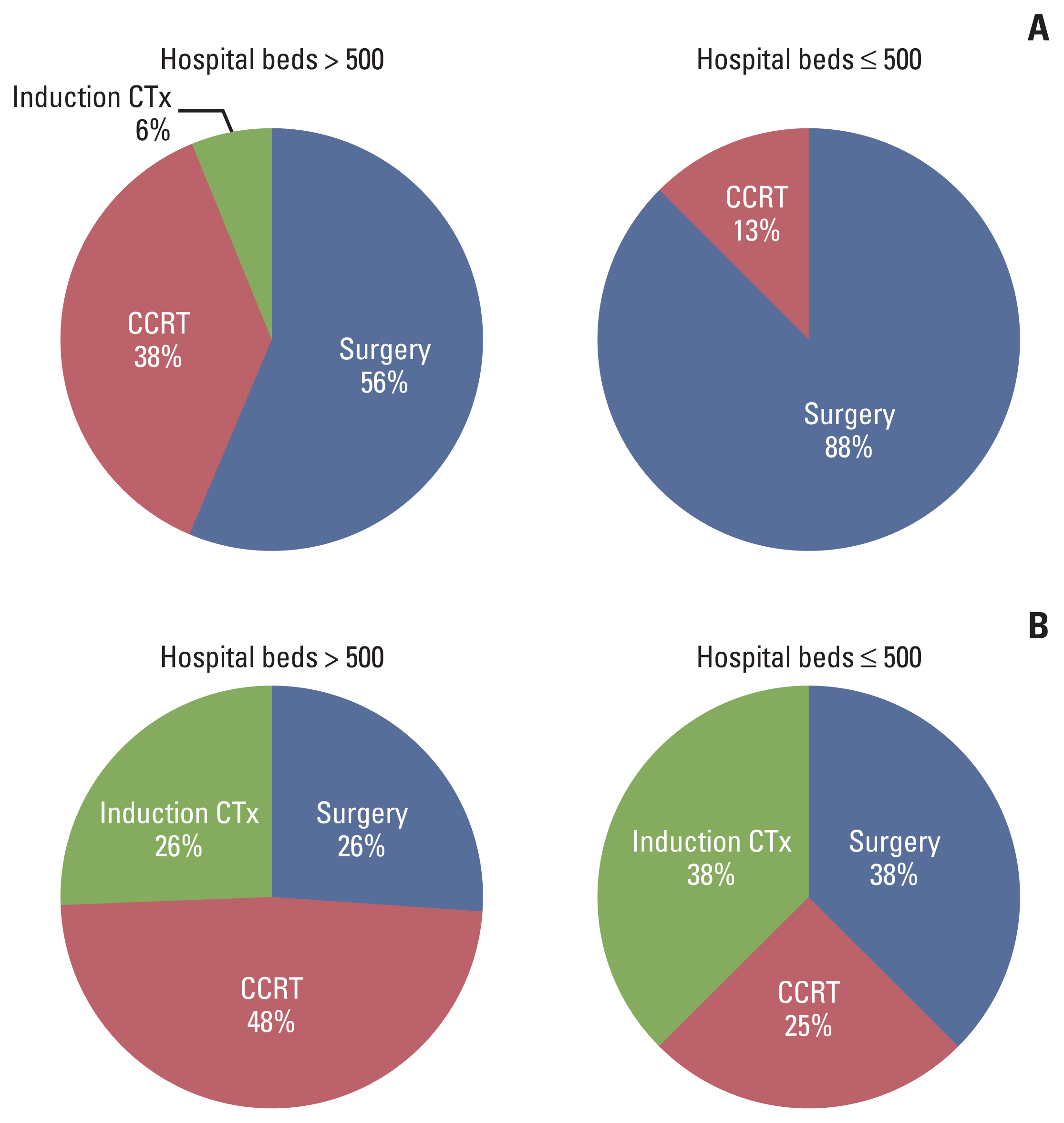
Table 1
Characteristics of the respondents
Table 2
Factors affecting the choice of treatment modalities in node-positive cases
| Characteristic | Surgerya) (n=96) | CCRTa) (n=88) | Induction chemotherapya) (n=39) | p-value |
|---|---|---|---|---|
| HPV status | ||||
| Positive | 37 (38.5) | 49 (55.7) | 25 (64.1) | 0.010 |
| Negative | 59 (61.5) | 39 (44.3) | 14 (35.9) | |
| Regional nodal metastasis | ||||
| N1 | 65 (67.7) | 35 (39.8) | 7 (17.9) | < 0.001 |
| N2–N3 | 31 (32.3) | 53 (60.2) | 32 (82.1) | |
| Department | ||||
| Otolaryngology | 29 (30.2) | 11 (12.5) | 13 (33.3) | < 0.001 |
| Radiation oncology | 37 (38.5) | 67 (76.1) | 16 (41.0) | |
| Medical oncology | 17 (17.7) | 8 (9.1) | 6 (15.4) | |
| Multidisciplinary team | 13 (13.5) | 2 (2.3) | 4 (10.3) | |
| Hospital beds | ||||
| > 1,000 | 27 (28.1) | 35 (39.8) | 12 (30.8) | 0.149 |
| > 500 and ≤ 1,000 | 57 (59.4) | 52 (59.1) | 24 (61.5) | |
| > 300 and ≤ 500 | 8 (8.3) | 1 (1.1) | 3 (7.7) | |
| N/A | 4 (4.2) | 0 ( | 0 ( | |
| Robotic surgery availability | ||||
| Available | 63 (65.6) | 59 (67.0) | 31 (79.5) | 0.280 |
| Not used | 27 (28.1) | 28 (31.8) | 7 (17.9) | |
| N/A | 6 (6.3) | 1 (1.1) | 1 (2.6) | |
| Consultation meeting | ||||
| Regular conference | 71 (74.0) | 61 (69.3) | 27 (69.2) | 0.781 |
| Meeting if necessary | 8 (8.3) | 10 (11.4) | 5 (12.8) | |
| Interdepartmental referral | 13 (13.5) | 17 (19.3) | 7 (17.9) | |
| N/A | 4 (4.2) | 0 | 0 | |




 PDF
PDF Citation
Citation Print
Print


 XML Download
XML Download