Introduction
Lung cancer is the leading cause of cancer-related death and the most common cancer in the world [
1]. With the continued aging of society, the proportion of older adult patients with lung cancer is gradually increasing globally [
2]. Of the 26,985 cases of newly diagnosed lung cancer in 2017 in Korea, 14,380 patients aged 70 years and above accounted for 53%, and 5,043 patients aged 80 years or above accounted for 18.7%, according to the Ministry of Health and Welfare Cancer registration statistics and the statistics of the causes of death from the National Statistical Office [
3]. In the United States, the overall mean age of diagnosis was 71 years [
4], and patients aged 80 years or above accounted for 17.8% of lung cancer cases, according to the Surveillance Epidemiology and End Results program in 2004 [
5].
However, decisions about treatment modalities for older adult patients with lung cancer are difficult to make in clinical practice. Older adult patients are under-represented in clinical trials; they have been excluded from the majority of clinical trials [
6–
9]. They may also not receive optimal treatment because they are generally more likely to have a poor performance status, co-existing comorbidities, drug interactions due to polypharmacy, and toxicity to chemotherapy. Moreover, because of age-related pessimism, the right medical decisions may not be made by doctors as well as patients and their families [
8].
Previous studies have retrospectively analyzed the clinical features and treatment modalities of lung cancer patients older than 65 years and found that the appropriate standard treatments, such as surgery or chemotherapy, for each stage of lung cancer were generally not administered in older adults with lung cancer [
10,
11]. In recent years, because of advances in targeted therapy and immunotherapy for non–small cell lung cancer (NSCLC), the proportion of older adult patients receiving treatment will increase [
2].
In this study, we retrospectively analyzed the clinical features and initial treatment modalities of extremely older lung cancer patients and compared their survival outcomes stratified by treatment. It has been predicted that the proportion of patients aged 80 years or above will increase over the coming decades, but studies on them are limited [
12]. Thus, we focused on older adult patients, especially those older than 80 years of age, with NSCLC. The results of this study will provide a therapeutic direction for treating patients aged 80 years or above. We aim to improve the quality of treatment for older adult lung cancer patients.
Results
A total of 6,576 patients were analyzed. Of them, 780 were aged 80 years or above and 5,796 were younger than 80 years (
Table 1). The male patients comprised 68.6% and 69.6% of the two groups, respectively (p=0.552). The proportion of ever-smokers was similar, 61.6% vs. 62.7% in each group (p=0.391). BMI was lower in the patients aged 80 years or above (21.80±3.33 vs. 23.18±3.43, p < 0.001). At the time of diagnosis, only 9.9% of the patients aged 80 years or above were asymptomatic as compared with 17.8% of the patients younger than 80 years (p < 0.001). In the patients aged 80 years or above, the three most common symptoms were cough, dyspnea, and sputum. The proportion of adenocarcinoma cases among the subtypes of NSCLC was lower among the patients aged 80 years or above (47.1% vs. 61.6%, p < 0.001). Conversely, the proportion of squamous cell carcinomas was higher among the patients aged 80 years or above (37.9% vs. 28.6%, p < 0.001). The proportion of patients with an ECOG performance status score greater than 2 was higher among the patients aged 80 years or above (33.2% vs. 10.4%, p < 0.001). There were fewer patients aged 80 years or above with stage I (20.9% vs. 31%, p < 0.001) and more patients with stage IV (53.6% vs. 41.9%, p < 0.001). The number of patients who received surgery (11.0%), concurrent chemo-radiation therapy (CCRT) (2.4%), or chemotherapy (14.4%) as their initial treatment was significantly lower among those aged 80 years or above (all, p < 0.001); the proportion of those who received radiation therapy (19.6%) was higher. Furthermore, 44% of patients did not receive any treatment among those aged 80 years or above.
Table 1
Baseline characteristics of the patients stratified by age
|
Age cohort |
Age ≥ 80 yr |
Age < 80 yr |
p-value |
|
No. of patients
|
780 |
5,796 |
|
|
Age (yr)
|
82 (81–84) |
67 (59–73) |
|
|
Male sex
|
535 (68.6) |
4,036 (69.6) |
0.552 |
|
Ever-smoker
|
469 (61.1) |
3,595 (62.7) |
0.391 |
|
BMI
|
21.80±3.33 |
23.18±3.43 |
< 0.001 |
|
Symptoms
|
|
Asymptomatic |
77 (9.9) |
1,033 (17.8) |
< 0.001 |
|
Cough |
278 (35.6) |
1,891 (32.6) |
0.093 |
|
Sputum |
203 (26.0) |
1,098 (18.9) |
< 0.001 |
|
Dyspnea |
224 (28.7) |
972 (16.8) |
< 0.001 |
|
Hoarseness |
11 (1.4) |
96 (1.7) |
0.610 |
|
Hemoptysis |
69 (8.8) |
326 (5.6) |
< 0.001 |
|
Weight loss |
51 (6.5) |
357 (6.2) |
0.680 |
|
Pain |
151 (19.4) |
1,027 (17.7) |
0.262 |
|
Histopathology
|
|
Squamous cell carcinoma |
296 (37.9) |
1,655 (28.6) |
< 0.001 |
|
Adenocarcinoma |
367 (47.1) |
3,572 (61.6) |
< 0.001 |
|
Large cell carcinoma |
4 (0.5) |
52 (0.9) |
0.273 |
|
NSCLC NOS |
58 (7.4) |
300 (5.2) |
0.009 |
|
Performance status
|
|
0, 1 |
320 (66.8) |
3,949 (89.6) |
< 0.001 |
|
2, 3, 4 |
159 (33.2) |
458 (10.4) |
< 0.001 |
|
Pulmonary function
|
|
FEV1 % predicted |
75.89±25.57 |
78.26±22.00 |
0.026 |
|
FVC % predicted |
71.49±20.59 |
80.95±19.63 |
< 0.001 |
|
Clinical stage of NSCLC
|
|
I |
163 (20.9) |
1,798 (31.0) |
< 0.001 |
|
II |
66 (8.5) |
485 (8.4) |
0.929 |
|
III |
126 (16.2) |
1,064 (18.4) |
0.133 |
|
IV |
418 (53.6) |
2,428 (41.9) |
< 0.001 |
|
Unknown |
7 (0.9) |
20 (0.3) |
0.024 |
|
Initial treatment of NSCLC
|
|
Surgery |
86 (11.0) |
2,304 (39.8) |
< 0.001 |
|
Surgery only |
81 (10.4) |
1,743 (30.1) |
< 0.001 |
|
Surgery and adjuvant therapy |
5 (0.6) |
561 (9.7) |
< 0.001 |
|
RT only |
153 (19.6) |
406 (7.0) |
< 0.001 |
|
CCRT |
19 (2.4) |
689 (11.9) |
< 0.001 |
|
Chemotherapy |
112 (14.4) |
1,380 (23.8) |
< 0.001 |
|
Best supportive care |
343 (44.0) |
854 (14.7) |
< 0.001 |
|
Unknown |
66 (8.5) |
163 (2.8) |
< 0.001 |

We compared the patient characteristics and initial treatment modalities for each clinical stage. Among stage I–II patients, the proportion of those who underwent surgery was lower among those aged 80 years and greater (31.3% vs. 84.6%, p < 0.001) (
S1 Table). The proportion of patients receiving radiation therapy was higher in those aged 80 years or above (26.4% vs. 5.2%, p < 0.001). In the older adult group, 29.5% of the patients did not receive any treatment.
Among stage III patients aged 80 years and greater, 7.1% underwent surgery, and the proportions of patients who received chemotherapy, radiation therapy alone, and CCRT were 11.8%, 23.6%, and 6.3%, respectively (
S2 Table); 43.3% did not receive any treatment. For stage III cancer, the proportion of patients receiving CCRT was lower among those aged 80 years or above (6.3% vs. 29.1%, p < 0.001); the proportion receiving radiation therapy only was higher (23.6% vs. 6.6%, p < 0.001).
For stage IV, the proportion of patients who were males was lower (62.5% vs. 70.9%, p=0.001) and the proportion of squamous cell carcinoma cases was higher (31.0% vs. 21.1%, p < 0.001) among those aged 80 years or above (
S3 Table). The proportion of patients who received chemotherapy was lower (21.8% vs. 45.7%, p < 0.001), and the proportion of patients who did not receive any treatment was higher (49.4% vs. 26%, p < 0.001). Of the 221 patients with stage IV adenocarcinoma, 182 underwent
EGFR mutation testing (
Table 2). A total of 25.3% men and 50.5% women were positive for
EGFR mutation status. Of the patients identified as
EGFR mutation-positive, 69.6% and 65.2% of men and women, respectively, were treated with EGFR-targeted therapy during the entire follow-up period. Only seven patients were identified as ALK-positive, and two of them were treated with ALK-targeted therapy.
Table 2
Characteristics of patients aged 80 years or above with stage IV adenocarcinoma
|
Total |
Men |
Women |
p-value |
|
No. of patients
|
221 |
115 |
106 |
|
|
Ever-smoker
|
95 (43.8) |
80 (70.8) |
15 (14.4) |
< 0.001 |
|
EGFR mutation (n=182)
|
|
Positive |
69 (37.9) |
23 (25.3) |
46 (50.5) |
0.001 |
|
Negative |
113 (62.1) |
68 (74.7) |
45 (49.5) |
0.001 |
|
EGFR inhibitor usage (n=69)
|
|
Positive |
46 (66.7) |
16 (69.6) |
30 (65.2) |
0.468 |
|
Negative |
23 (33.3) |
7 (30.4) |
16 (34.8) |
0.468 |
|
ALK mutation (n=123)
|
|
Positive |
7 (5.7) |
2 (3.0) |
5 (8.8) |
0.314 |
|
Negative |
116 (94.3) |
64 (97.0) |
52 (91.2) |
0.314 |
|
ALK inhibitor usage (n=7)
|
|
Positive |
2 (28.6) |
0 |
2 (40.0) |
0.4 |
|
Negative |
5 (71.4) |
2 (100) |
3 (60.0) |
0.4 |

Using Cox proportional hazard models, we identified the factors underlying mortality in patients aged 80 years or above with stage I–II and stage IV NSCLC. In the multivariable analysis, BMI, clinical stage (I or II), and initial treatment modalities were identified as factors that affected mortality in stage I–II patients (
Table 3). A higher BMI was associated with a lower risk with a hazard ratio (HR) of 0.912 (p=0.027); stage II was associated with a higher risk of mortality (2.499 times that of stage I, p=0.001). Compared to surgery, the best supportive care significantly increased mortality with an HR of 4.355 (p < 0.001), whereas radiation treatment did not show a significant difference with an HR of 1.597 (p=0.218). For stage IV, the multivariable analysis revealed that male sex, BMI, histopathologic type, and chemotherapy were identified as factors that affected mortality (
Table 4). Male sex increased mortality with an HR of 1.693 (p=0.001). A higher BMI was also a significant protective factor with an HR of 0.939 (p=0.004). Compared with squamous cell carcinoma, large cell carcinoma, and other unspecified forms of NSCLC showed increased mortality with an HR of 1.960 (p=0.007). Compared with chemotherapy, the best supportive care resulted in increased mortality with an HR of 1.936 (p < 0.001).
Table 3
Risk factors of mortality in patients aged 80 years or above with stage I–II NSCLC using Cox proportional hazards models
|
Univariate analysis |
Multivariate analysis |
|
|
|
Hazard ratio |
95% CI |
p-value |
Hazard ratio |
95% CI |
p-value |
|
Age
|
1.102 |
1.029–1.180 |
0.006 |
1.048 |
0.928–1.183 |
0.450 |
|
|
Male sex
|
1.820 |
1.066–3.108 |
0.028 |
1.374 |
0.643–2.934 |
0.412 |
|
|
Ever-smoker
|
1.731 |
1.076–2.786 |
0.024 |
0.962 |
0.456–2.026 |
0.918 |
|
|
BMI
|
0.871 |
0.819–0.927 |
< 0.001 |
0.912 |
0.840–0.990 |
0.027 |
|
|
Histopathology
|
|
|
< 0.001 |
|
|
0.467 |
|
|
Squamous cell carcinoma (ref) |
1.000 |
|
|
1.000 |
|
|
|
|
Adenocarcinoma |
0.441 |
0.279–0.697 |
< 0.001 |
0.668 |
0.352–1.268 |
0.217 |
|
|
Others |
1.411 |
0.766–2.600 |
0.269 |
0.919 |
0.463–1.822 |
0.809 |
|
|
FEV1% predicted
|
0.986 |
0.976–0.995 |
0.004 |
0.991 |
0.979–1.004 |
0.157 |
|
|
Clinical stage of NSCLC
|
|
|
< 0.001 |
|
|
0.001 |
|
|
I (ref) |
1.000 |
|
|
1.000 |
|
|
|
|
II |
3.269 |
2.176–4.911 |
< 0.001 |
2.499 |
1.454–4.294 |
0.001 |
|
|
Treatment
|
|
|
< 0.001 |
|
|
< 0.001 |
|
|
Surgery (ref) |
1.000 |
|
|
1.000 |
|
|
|
|
Radiation therapy |
2.681 |
1.455–4.940 |
0.002 |
1.597 |
0.759–3.360 |
0.218 |
|
|
Best supportive care |
8.248 |
4.683–14.528 |
< 0.001 |
4.355 |
2.152–8.816 |
< 0.001 |

Table 4
Risk factors of mortality in patients with aged 80 years or above with stage IV NSCLC using Cox proportional hazards models
|
Univariate analysis |
Multivariate analysis |
|
|
|
Hazard ratio |
95% CI |
p-value |
Hazard ratio |
95% CI |
p-value |
|
Age
|
1.005 |
0.974–1.037 |
0.753 |
0.987 |
0.951–1.025 |
0.494 |
|
|
Male sex
|
1.574 |
1.278–1.938 |
< 0.001 |
1.693 |
1.227–2.335 |
0.001 |
|
|
Ever-smoker
|
1.342 |
1.096–1.644 |
0.004 |
0.872 |
0.640–1.188 |
0.384 |
|
|
BMI
|
0.931 |
0.900–0.962 |
< 0.001 |
0.939 |
0.899–0.980 |
0.004 |
|
|
Histopathology
|
|
|
< 0.001 |
|
|
< 0.001 |
|
|
Squamous cell carcinoma (ref) |
1.000 |
|
|
1.000 |
|
|
|
|
Adenocarcinoma |
0.608 |
0.485–0.762 |
< 0.001 |
0.912 |
0.592–1.113 |
0.196 |
|
|
Others |
1.720 |
1.184–2.500 |
0.004 |
1.960 |
1.205–3.188 |
0.007 |
|
|
Treatment
|
|
|
< 0.001 |
|
|
< 0.001 |
|
|
Chemotherapy (ref) |
1.000 |
|
|
1.000 |
|
|
|
|
Best supportive care |
2.274 |
1.733–2.985 |
< 0.001 |
1.936 |
1.421–2.638 |
< 0.001 |

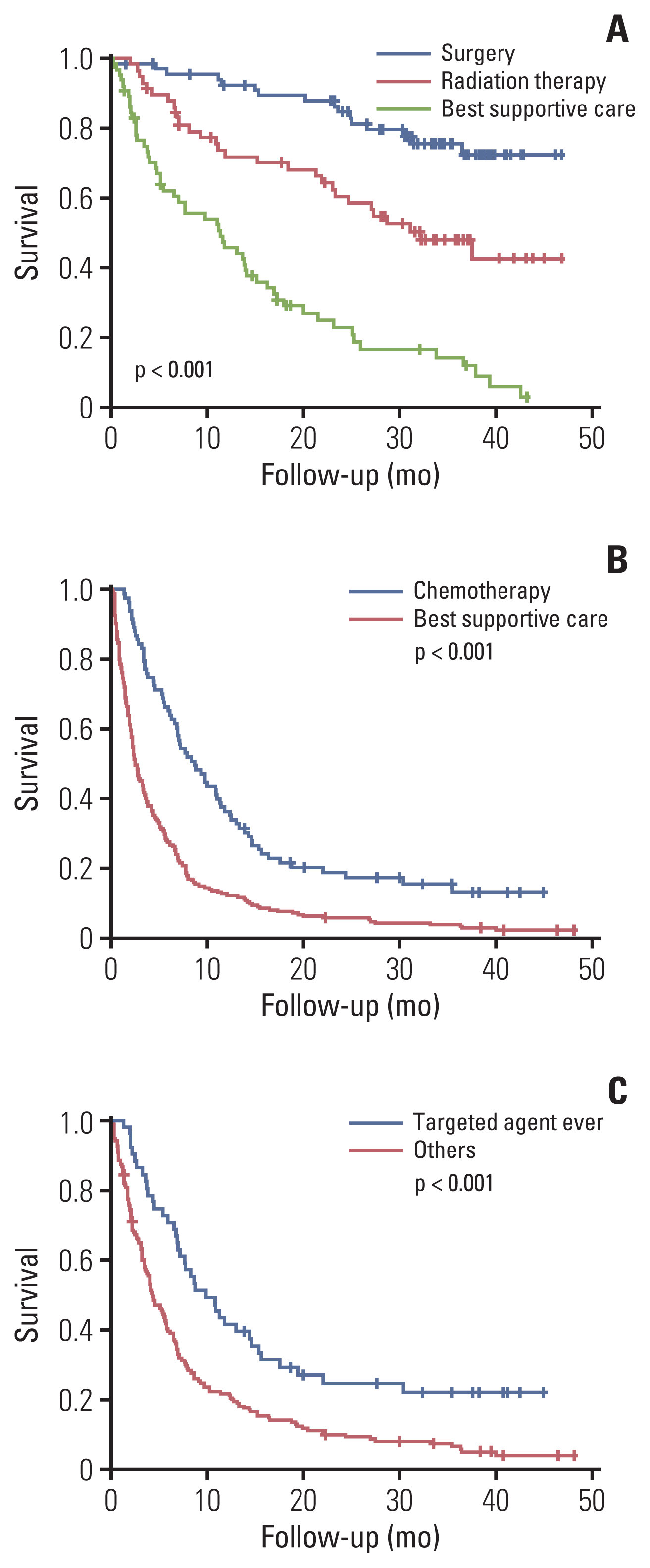
We compared the overall survival among the patients aged 80 years or above with stage I–II who received surgery, radiation therapy, and best supportive care using the Kaplan-Meier method and log-rank analysis. Significant overall survival differences were identified among the three groups (median survival, not reached, 32.2 months, and 11.43 months, respectively; p < 0.001) (
Fig. 1A). Among the stage IV patients aged 80 years or above, a survival advantage was identified in those who received chemotherapy over those who received the best supportive care (median survival, 8.63 months vs. 2.5 months; p < 0.001) (
Fig. 1B). We compared the overall survival of the patients with stage IV adenocarcinomas who were treated with and without a targeted agent. A significant improvement in survival was observed in the patients treated with a targeted agent (median survival, 9.9 months vs. 4.3 months; p < 0.001) (
Fig. 1C).
Fig. 1
Overall survival of stage I–II (A) and stage IV (B) non–small cell lung cancer (NSCLC) patients aged 80 years or older stratified by initial treatment modalities. Overall survival of patients with stage IV adenocarcinoma treated with and without a targeted agent (C). (A) In patients aged ≥ 80 years, surgery and radiation therapy resulted in longer patient survival among those with the resectable stage (I–II) than the best supportive care (median survival, not reached [surgery] vs. 32.2 months [radiation] vs. 11.43 months [best supportive care]). (B) Chemotherapy resulted in longer survival of patients with advanced-stage (IV) disease than best supportive care (median survival, 8.63 months vs. 2.5 months). (C) Stage IV adenocarcinoma patients who received targeted therapy had better survival than those who did not (median survival, 9.0 months vs. 4.3 months).

We performed subgroup analysis of patients with ECOG performance status 0–1 because those with poor performance statuses tended not to receive treatment. Using the Kaplan-Meier method and the log-rank test, improvement in survival was confirmed in actively treated patients with stage I–II and stage IV (
S4 and
S5 Figs.). Multivariable Cox analysis revealed that the patients in the best supportive care group had 4.52 times higher mortality than those who underwent surgery in the stage I–II group; radiation therapy did not show any significant differences (
S6 Table). For stage IV, the patients who did not receive any treatment showed higher mortality than those who received chemotherapy (HR, 2.022; p=0.004) (
S7 Table).
Discussion
This retrospective study identified the baseline characteristics and treatment outcomes stratified by the initial treatment modalities in NSCLC patients aged 80 years or above. Compared with patients younger than 80 years, the older adult patients were more likely to have more symptoms related to lung cancer, were initially diagnosed with advanced-stage disease, and did not receive any treatment despite being early-stage lung cancer cases. However, this study confirmed that the patients who received standard treatment in both the early and advanced stages showed clear improvements in overall survival compared to those who did not receive any treatment. Based on this observation, we should not give up on treatment among patients aged 80 years or above solely based on their chronological age. We need to select the best possible treatment options, taking into account each patient’s condition.
We compared surgery, radiation therapy, and the best supportive care using the Kaplan-Meier method and the log-rank test in patients aged 80 years or above in the stage I–II group; the most significant improvement in overall survival was observed in the surgery group followed by the radiation therapy group. Multivariate Cox analysis revealed that the radiation therapy group had a 1.5 times increased risk of death as compared with the surgery group, but it was not statistically significant. The number of patients aged 80 years or above undergoing radiation therapy has been gradually increasing globally. According to a study published in 2007, 47% of patients with NSCLC aged 80 years or above received radiation therapy during the overall treatment period [
14]. According to a study published by Lee et al. [
15] in 2018, the rate of stereotactic body radiation therapy (SBRT) for early-stage lung cancer in Korea increased from 9.4 to 28.6% from 2008 to 2016, and SBRT was selected as an alternative to surgery if high-risk complications were predicted after surgery. In the same study, the overall durations of survival of patients aged 80 years or above with early-stage NSCLC after surgery and SBRT were 35.5 and 56.4 months, respectively, confirming that overall survival was inferior for SBRT. However, because of the retrospective study design, selection bias may have existed in the SBRT group, since patients who were contraindicated for surgery were selected. In the current study, we corrected for factors, such as age, sex, smoking history, BMI, histopathologic types, basal pulmonary function, and stage, using multivariate Cox analysis; the results showed no significant increase in the mortality risk in the radiation therapy group. Another retrospective study compared surgery and radiation therapy among patients with stage I lung cancer using an intention-to-treat analysis [
16]. In that paper, multivariable Cox modeling revealed inferior overall survival in the SBRT group to that of the surgery group. After comparing cancer-specific survival, it was confirmed that there was no difference. Therefore, it is better to perform surgery during the resectable stage, if possible, but if the patient has a condition that makes it difficult to tolerate surgery, radiation therapy may extend the survival of patients.
Following the initial treatments of stage IV NSCLC, the overall survival of the chemotherapy group was superior to that of the best supportive care group. A previous study compared the effects of systemic chemotherapy, including platinum doublet treatment, in patients with advanced NSCLC younger and those older than 80 years [
5]. There was no difference between the progression-free survival and overall survival after chemotherapy stratified by age group. Thus, chemotherapy was considered to be equally beneficial among older adult and younger patients. Of the 83 patients in the current study who chose chemotherapy as their initial treatment, 44 were treated with a targeted agent, and 39 were treated with conventional chemotherapy.
The stage IV adenocarcinoma patients were stratified into two groups based on whether they received targeted therapy or not; their survival rates were improved by 9.9 and 4.3 months, respectively. Previous studies have reported that targeted treatment for EGFR-positive patients older than 80 years can reverse poor prognosis due to a poor performance status [
2], and according to the Korean Association for Lung Cancer survey in Korea in 2016, EGFR-positive cases are confirmed in approximately 36.8% of all stage adenocarcinomas, 51.2% of women, and 26.6% of men [
13]. Therefore, it is essential to perform mutation tests for stage IV cases, and we expect to improve the overall survival of patients using targeted agents when it is possible. Interestingly, the proportion of squamous cell carcinoma cases increases with age, while that of adenocarcinoma cases decreases. Compared with those younger than 80 years, the percentage of squamous cell carcinoma cases in older adult patients is higher. The most common histopathological type in 1997 was squamous cell carcinoma. In 2005, adenocarcinoma became the most common, and this change has been explained by differences in cigarette exposure, occupational risks, and/or air pollution [
13,
17]. However, 47.1% of adenocarcinoma patients were still reported as EGFR-positive, even with the smaller percentage of adenocarcinoma in older adults, and especially during the advanced stage of the cancer. The importance of obtaining a mutation profile needs to be emphasized because the quality of life is less affected by this treatment.
The life expectancies of 80-year-old men and women are 87.3 and 89.0 years, respectively [
5], and treatment can extend the lives of patients for as long as 10 years, even if they are diagnosed with lung cancer after 80 years of age, especially among those with early-stage lung cancer. In patients aged 80 years or above, the higher rate of complaints about their symptoms at the time of diagnosis and the stage IV ratio highlights the utility of check-ups for lung cancer after 80 years.
This study design is retrospective, and we did not have data on patients’ comorbidities and cancer-specific mortality. To overcome this limitation, we analyzed patient mortality using multivariate Cox analysis and excluded other factors that affected mortality. We identified survival outcomes stratified by their initial treatment modalities. For example, low BMI was related to higher mortality in early- and advanced-stage patients aged 80 years or above; older patients with insufficient nutritional support and cachexia had poorer survival outcomes. After excluding factors, such as BMI, sex, and pulmonary function, among others, in this analysis, the patients with active treatment showed improved survival. In the future, based on available information about the patient comorbidities or cancer-specific mortality, complementary studies will be needed. Patients with a poor performance status tended not to receive treatment, and because of this, their prognoses were worse than those of good performance status patients. To take this bias into account, we performed an exclusive subgroup analysis including patients with ECOG performance status 0–1. As a result, survival outcome improvement was also confirmed in actively treated patients in both early- and advanced-stage good performance status patients. Therefore, standard treatment should be considered, especially in patients with good performance status.
In the early stages of NSCLC in patients older than 80 years, active treatments such as surgery or radiation therapy improved overall survival. Chemotherapy improved the overall survival of patients with advanced-stage NSCLC; targeted therapy also improved their survival rate, so mutation testing is essential in Asia, where the rate of EGFR mutations is high. Rather than simply selecting conservative treatment due to a patient’s chronologic age, it is better to assess each patient’s performance status and individual condition and provide the appropriate active treatment.





 PDF
PDF Citation
Citation Print
Print


 XML Download
XML Download