Introduction
Materials and Methods
1. Patients
2. Definitions of platelet-related indices
3. Statistical analysis
Results
1. Baseline characteristics
Table 1
| Characteristic | Total | Validation datasets | Training datasets | p-value |
|---|---|---|---|---|
| No. | 527 | 156 | 371 | |
| Type | ||||
| GBC | 122 (23.1) | 37 (23.7) | 85 (22.9) | 0.953 |
| ECC | 296 (56.2) | 86 (55.1) | 210 (56.6) | |
| ICC | 109 (20.7) | 33 (21.2) | 76 (20.5) | |
| Age (yr) | ||||
| ≤ 60 | 233 (44.3) | 64 (41.3) | 169 (45.6) | 0.423 |
| > 60 | 293 (55.7) | 91 (58.7) | 202 (54.4) | |
| Sex | ||||
| Female | 235 (44.6) | 76 (48.7) | 159 (42.9) | 0.254 |
| Male | 292 (55.4) | 80 (51.3) | 212 (57.1) | |
| Blood type | ||||
| AB | 35 (6.7) | 10 (6.5) | 25 (6.8) | 0.752 |
| A | 114 (21.7) | 38 (24.5) | 76 (20.5) | |
| B | 214 (40.8) | 59 (38.1) | 155 (41.9) | |
| O | 162 (30.9) | 48 (31.0) | 114 (30.8) | |
| Fever | ||||
| No | 451 (85.6) | 131 (84.0) | 320 (86.3) | 0.586 |
| Yes | 76 (14.4) | 25 (16.0) | 51 (13.7) | |
| Emaciation | ||||
| No | 259 (49.6) | 71 (46.1) | 188 (51.1) | 0.346 |
| Yes | 263 (50.4) | 83 (53.9) | 180 (48.9) | |
| Debilitation | ||||
| No | 467 (89.0) | 135 (86.5) | 332 (90.0) | 0.320 |
| Yes | 58 (11.0) | 21 (13.5) | 37 (10.0) | |
| Drink | ||||
| No | 402 (76.6) | 120 (76.9) | 282 (76.4) | 0.991 |
| Yes | 123 (23.4) | 36 (23.1) | 87 (23.6) | |
| Fat liver | ||||
| No | 453 (91.9) | 135 (91.2) | 318 (92.2) | 0.859 |
| Yes | 40 (8.1) | 13 (8.8) | 27 (7.8) | |
| ALT (U/L) | ||||
| ≤ 100 | 298 (56.7) | 91 (58.3) | 207 (55.9) | 0.683 |
| > 100 | 228 (43.3) | 65 (41.7) | 163 (44.1) | |
| AST (U/L) | ||||
| ≤ 100 | 351 (69.8) | 103 (69.6) | 248 (69.9) | > 0.99 |
| > 100 | 152 (30.2) | 45 (30.4) | 107 (30.1) | |
| GGT (U/L) | ||||
| ≤ 200 | 232 (46.0) | 70 (47.3) | 162 (45.5) | 0.788 |
| > 200 | 272 (54.0) | 78 (52.7) | 194 (54.5) | |
| ALP (U/L) | ||||
| ≤ 200 | 230 (45.6) | 68 (45.9) | 162 (45.5) | > 0.99 |
| > 200 | 274 (54.4) | 80 (54.1) | 194 (54.5) | |
| ALB (g/L) | ||||
| ≤ 35 | 112 (21.3) | 34 (21.8) | 78 (21.1) | 0.959 |
| > 35 | 413 (78.7) | 122 (78.2) | 291 (78.9) | |
| TBIL (μmol/L)a) | ||||
| ≤ 106 | 323 (61.3) | 99 (63.5) | 224 (60.4) | 0.572 |
| > 106 | 204 (38.7) | 57 (36.5) | 147 (39.6) | |
| CA 19-9 (U/mL) | ||||
| ≤ 100 | 256 (51.0) | 70 (48.3) | 186 (52.1) | 0.497 |
| > 100 | 246 (49.0) | 75 (51.7) | 171 (47.9) | |
| Tumor differentiationb) | ||||
| Poor | 51 (10.1) | 20 (13.2) | 31 (8.8) | 0.266 |
| Moderate | 262 (52.0) | 73 (48.3) | 189 (53.5) | |
| Well | 191 (37.9) | 58 (38.4) | 133 (37.7) | |
| Radical cure | ||||
| No | 200 (38.3) | 64 (41.6) | 136 (37.0) | 0.375 |
| Yes | 322 (61.7) | 90 (58.4) | 232 (63.0) | |
| T category | ||||
| Tis | 9 (1.7) | 2 (1.3) | 7 (1.9) | 0.362 |
| 1 | 130 (24.7) | 36 (23.1) | 94 (25.3) | |
| 2 | 153 (29.0) | 55 (35.3) | 98 (26.4) | |
| 3 | 196 (37.2) | 52 (33.3) | 144 (38.8) | |
| 4 | 39 (7.4) | 11 (7.1) | 28 (7.5) | |
| Nodal involvement | ||||
| No | 364 (69.3) | 113 (73.4) | 251 (67.7) | 0.234 |
| Yes | 161 (30.7) | 41 (26.6) | 120 (32.3) | |
| TNM stage | ||||
| 0 | 9 (1.7) | 2 (1.3) | 7 (1.9) | 0.737 |
| I | 179 (34.0) | 57 (36.5) | 122 (32.9) | |
| II | 174 (33.0) | 47 (30.1) | 127 (34.2) | |
| III | 165 (31.3) | 50 (32.1) | 115 (31.0) | |
| Tumor size (cm)a) | ||||
| ≤ 2.6 | 303 (60.2) | 87 (58.8) | 216 (60.8) | 0.741 |
| > 2.6 | 200 (39.8) | 61 (41.2) | 139 (39.2) | |
| Postoperative chemotherapy | ||||
| No | 353 (76.2) | 103 (73.6) | 250 (77.4) | 0.441 |
| Yes | 110 (23.8) | 37 (26.4) | 73 (22.6) | |
Values are presented as number (%). ALB, albumin; ALP, alkaline phosphatase; ALT, alanine aminotransferase; AST, aspartate aminotransferase; CA 19-9, carbohydrate antigen 19-9; ECC, extrahepatic cholangiocarcinoma; GBC, gallbladder cancer; GGT, γ-glutamyl transpeptidase; ICC, intrahepatic cholangiocarcinoma; TBIL, total bilirubin.
a) Cut-off values of TBIL (106 μmol/L) and tumor sice (2.6 cm) were determined by ‘maxstat’ R package,
b) Tumor differentiation was judged by pathologists. Poor includes undifferentiated or poorly differentiated, which means disordered arrangement of cancer cells, high cellular atypia and high proportion of cancer cells in division phase. Well means almost normal arrangement of cancer cells, low cellular atypia and low proportion of cancer cells in division phase. Moderate means somewhere in between.
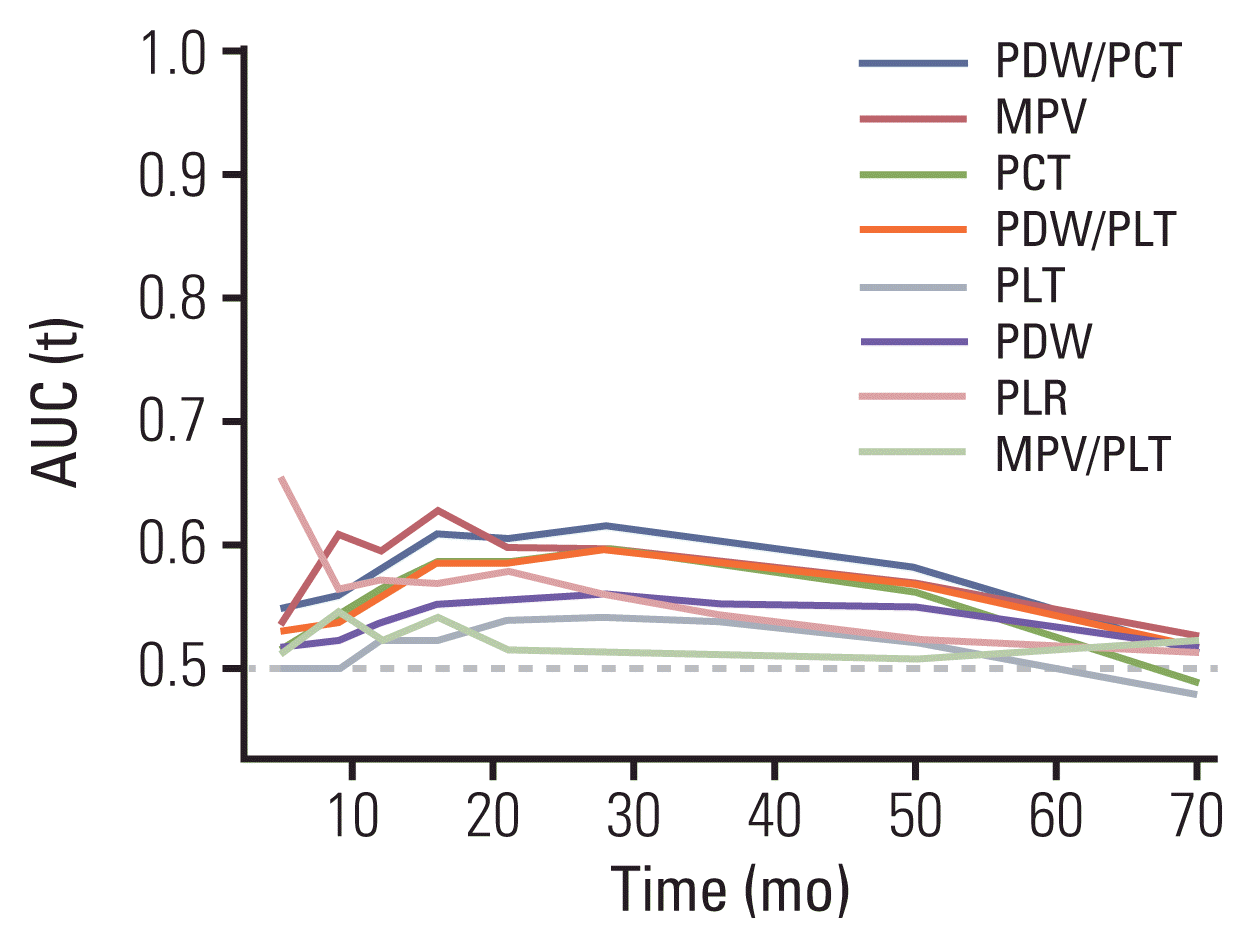
2. Comparison of the prognostic efficacy of platelet indices
Table 2
| Univariate analysis | Multivariate analysis | |||
|---|---|---|---|---|
|
|
|
|||
| HR (95% CI) | p-value | HR (95% CI) | p-value | |
| Type | ||||
|
|
||||
| GBC | Reference | |||
|
|
||||
| ECC | 0.97 (0.74–1.27) | 0.799 | ||
|
|
||||
| ICC | 1.12 (0.81–1.54) | 0.496 | ||
|
|
||||
| Age (yr) | ||||
|
|
||||
| ≤ 60 | Reference | |||
|
|
||||
| > 60 | 1.21 (0.97–1.51) | 0.087 | ||
|
|
||||
| Sex | ||||
|
|
||||
| Female | Reference | |||
|
|
||||
| Male | 0.97 (0.78–1.21) | 0.785 | ||
|
|
||||
| Blood typea) | ||||
|
|
||||
| AB | Reference | |||
|
|
||||
| A | 0.67 (0.42–1.07) | 0.091 | ||
|
|
||||
| B | 0.85 (0.56–1.31) | 0.467 | ||
|
|
||||
| O | 0.85 (0.55–1.32) | 0.467 | ||
|
|
||||
| Fever | ||||
|
|
||||
| No | Reference | |||
|
|
||||
| Yes | 1.02 (0.75–1.39) | 0.902 | ||
|
|
||||
| Emaciation | ||||
|
|
||||
| No | Reference | |||
|
|
||||
| Yes | 1.35 (1.08–1.68) | 0.007b) | ||
|
|
||||
| Debilitation | ||||
|
|
||||
| No | Reference | |||
|
|
||||
| Yes | 1.10 (0.78–1.55) | 0.582 | ||
|
|
||||
| Drink | ||||
|
|
||||
| No | Reference | |||
|
|
||||
| Yes | 0.91 (0.70–1.18) | 0.491 | ||
|
|
||||
| Fat liver | ||||
|
|
||||
| No | Reference | |||
|
|
||||
| Yes | 1.19 (0.80–1.77) | 0.393 | ||
|
|
||||
| ALT (U/L) | ||||
|
|
||||
| ≤ 100 | Reference | |||
|
|
||||
| > 100 | 1.17 (0.94–1.45) | 0.167 | ||
|
|
||||
| AST (U/L) | ||||
|
|
||||
| ≤ 100 | Reference | |||
|
|
||||
| > 100 | 1.17 (0.92–1.48) | 0.198 | ||
|
|
||||
| GGT (U/L) | ||||
|
|
||||
| ≤ 200 | Reference | |||
|
|
||||
| > 200 | 1.23 (0.98–1.53) | 0.073 | ||
|
|
||||
| ALP (U/L) | ||||
|
|
||||
| ≤ 200 | Reference | |||
|
|
||||
| > 200 | 1.40 (1.12–1.75) | 0.003b) | ||
|
|
||||
| ALB (g/L) | ||||
|
|
||||
| ≤ 35 | Reference | |||
|
|
||||
| > 35 | 0.64 (0.50–0.81) | < 0.001b) | ||
|
|
||||
| TBIL (μmol/L) | ||||
|
|
||||
| ≤ 106 | Reference | |||
|
|
||||
| > 106 | 1.61 (1.30–2.01) | < 0.001b) | ||
|
|
||||
| CA19-9 (U/mL) | ||||
|
|
||||
| ≤ 100 | Reference | |||
|
|
||||
| > 100 | 2.01 (1.60–2.53) | < 0.001b) | 1.60 (1.25–2.05) | < 0.001b) |
|
|
||||
| Tumor differentiation | ||||
|
|
||||
| Poor | Reference | |||
|
|
||||
| Moderate | 0.68 (0.47–0.98) | 0.040b) | 0.72 (0.59–0.87) | < 0.001b) |
|
|
||||
| Well | 0.44 (0.30–0.65) | < 0.001b) | ||
|
|
||||
| Radical cure | ||||
|
|
||||
| No | Reference | |||
|
|
||||
| Yes | 0.40 (0.32–0.50) | < 0.001b) | 0.47 (0.37–0.60) | < 0.001b) |
|
|
||||
| T category | ||||
|
|
||||
| Tis | Reference | |||
|
|
||||
| 1 | 5.38 (0.75–38.71) | 0.095 | ||
|
|
||||
| 2 | 6.43 (0.90–46.14) | 0.064 | ||
|
|
||||
| 3 | 9.03 (1.26–64.60) | 0.028a) | ||
|
|
||||
| 4 | 6.07 (0.82–45.13) | 0.078 | ||
|
|
||||
| Nodal involvement | ||||
|
|
||||
| No | Reference | |||
|
|
||||
| Yes | 1.99 (1.58–2.50) | < 0.001b) | 1.68 (1.31–2.15) | < 0.001b) |
|
|
||||
| Tumor size (cm) | ||||
|
|
||||
| ≤ 2.6 | Reference | |||
|
|
||||
| > 2.6 | 0.90 (0.72–1.13) | 0.364 | ||
|
|
||||
| Postoperative chemotherapy | ||||
|
|
||||
| No | Reference | |||
|
|
||||
| Yes | 0.93 (0.71–1.22) | 0.624 | ||
|
|
||||
| MPV (fl) | ||||
|
|
||||
| ≤ 8.1 | Reference | |||
|
|
||||
| > 8.1 | 1.52 (1.19–1.95) | < 0.001b) | 1.33 (1.01–1.75) | 0.045b) |
|
|
||||
| PDW/PCT | ||||
|
|
||||
| ≤ 190 | Reference | |||
|
|
||||
| > 190 | 0.65 (0.52–0.82) | < 0.001b) | 0.78 (0.61–1.00) | 0.046b) |
ALB, albumin; ALP, alkaline phosphatase; ALT, alanine aminotransferase; AST, aspartate aminotransferase; BTC, biliary tract cancer; CA19-9, carbohydrate antigen 19-9; CI, confidence interval; ECC, extrahepatic cholangiocarcinoma; GBC, gallbladder cancer; GGT, γ-glutamyl transpeptidase; HR, hazard ratio; ICC, intrahepatic cholangiocarcinoma; MPV, mean platelet volume; OS, overall survival; PCT, plateletocrit; PDW, platelet distribution width; TBIL, total bilirubin.
3. Relationships between PDW/PCT, MPV, and clinicopathological features
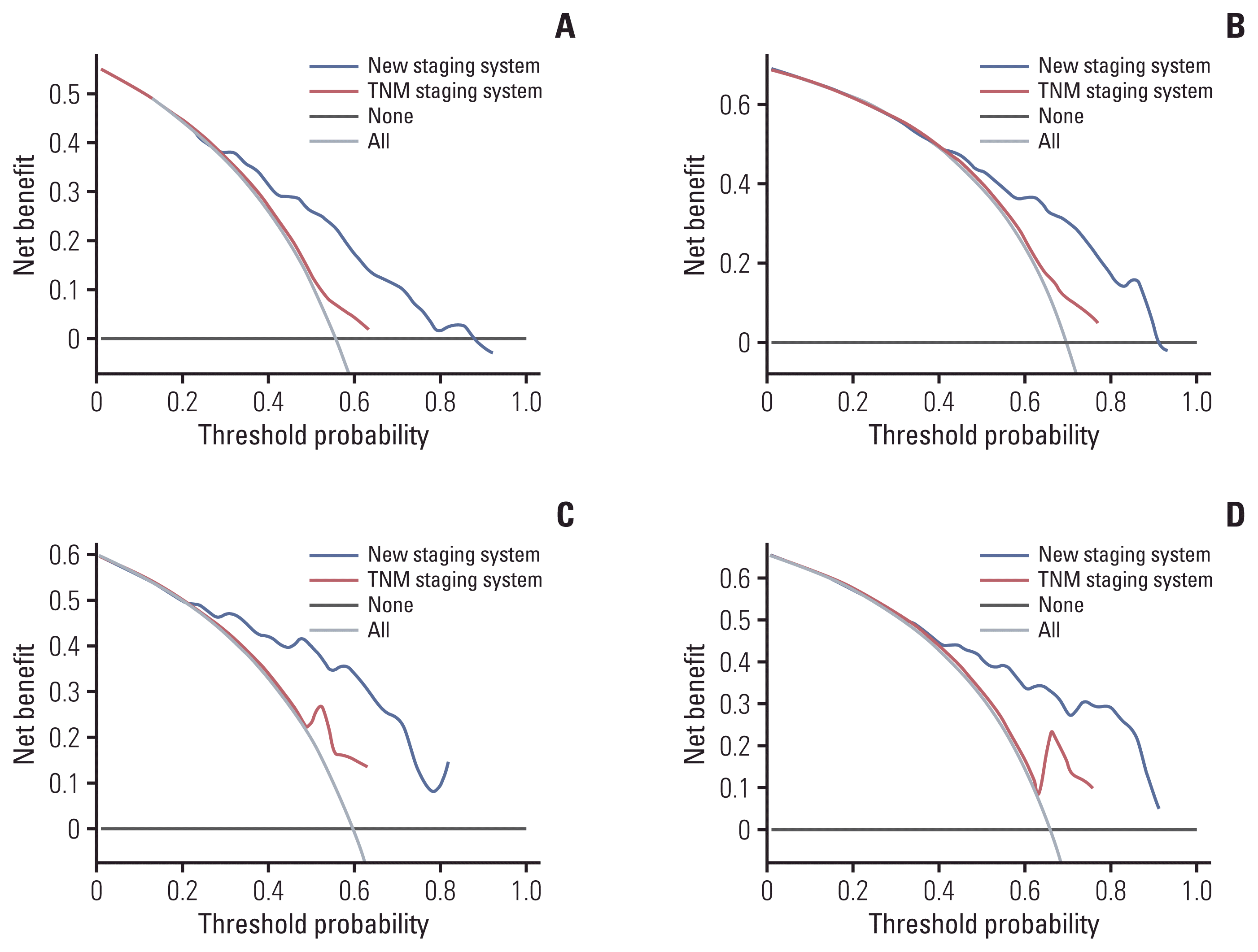
4. Development and validation of a novel staging system
Fig. 2
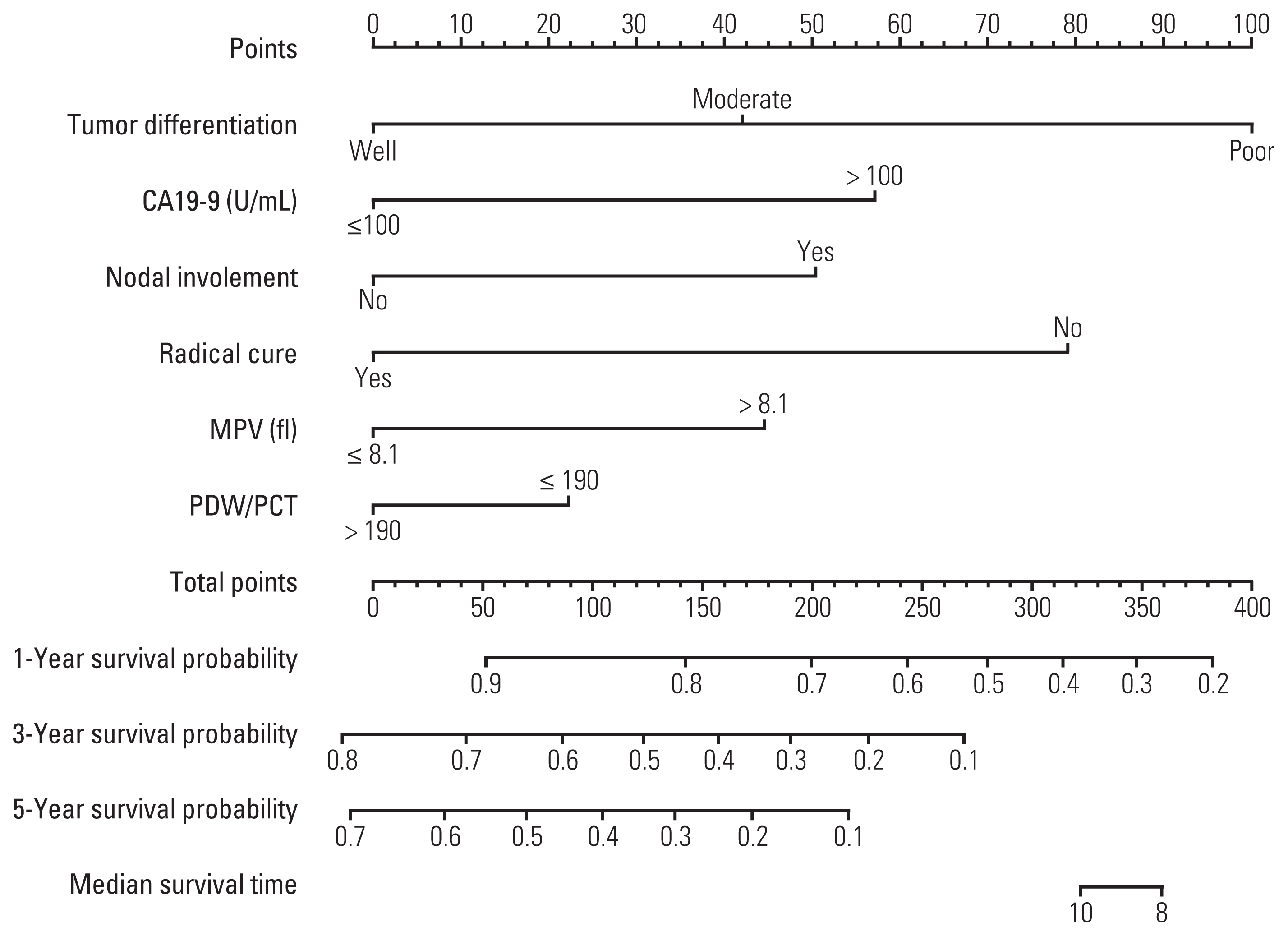
Fig. 3
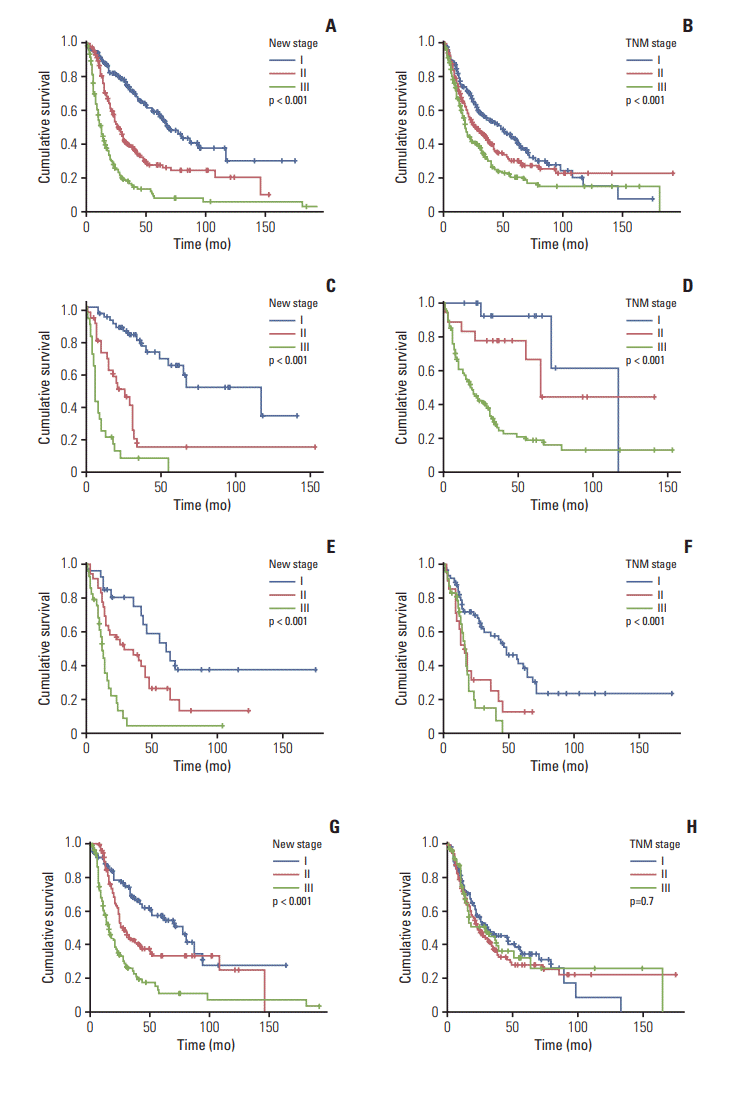
Fig. 4




 PDF
PDF Citation
Citation Print
Print


 XML Download
XML Download