Abstract
Purpose
This study aimed to evaluate the risk of cervical cancer diagnosed within 1 year after the last of multiple consecutive normal Papanicolau (Pap) tests.
Materials and Methods
The database of the National Health Insurance Service was used. We obtained Pap test data for 11,052,116 women aged 30–79 between 2007–2012. The cumulative incidence rates and 5-year overall survival rates of cervical cancer diagnosed within 1 year after the last normal Pap test were compared between women with one (N1), two (N2), and three consecutive normal Pap tests (N3). Women who did not receive a Pap test during the study period were assigned in the N0 group.
Results
The 1-year cumulative incidence rates of cervical cancer were 58.9, 24.6, 20.3, and 14.2 per 105 in the N0, N1, N2, and N3 groups, respectively. Compared to the N1 group, the risk of cervical cancer diagnosed within 1 year of the last normal Pap test decreased by 17% (relative risk [RR], 0.825; 95% confidence interval [CI], 0.716 to 0.951) in the N2 group and 42% (RR, 0.578; 95% CI, 0.480 to 0.695) in the N3 group. However, the 5-year survival rate in women diagnosed with cervical cancer within 1 year of the last normal Pap test in the N3 group was not higher than that of the N1 group (79.6% vs. 81.3%, p=0.706).
Cervical cancer is a common female cancer in developed countries, and there were 885,193 new cases and 311,365 deaths from cervical cancer worldwide in 2018 [1]. Cytology-based screening for cervical cancer has led to a remarkable reduction in incidence worldwide [2–4]. The Papanicolaou (Pap) test was first introduced as a method of cytology-based screening in 1941 [5], since then national screening programs using the Pap test with 3- to 5-year intervals is the most common strategy in many countries [6,7]. The majority of cervical cancer deaths occur in women who have never received a Pap test, but some occur in women who have had recently confirmed normal Pap results [8–10]. While these studies suggest that a large proportion (49%–72%) of patients with cervical cancer had either not been screened or had been improperly screened, as many as 30%–50% of women with cervical cancer had had a normal Pap result in the 3–5 years before their cervical cancer diagnosis. These findings not only suggest that it is necessary to increase participation in cervical cancer screening, but also that technical improvements in the Pap test may only result in a potential reduction in false-negative results and mortality from cervical cancer.
Several meta-analyses have reported low sensitivities of the Pap test, ranging from 37%–87% [11,12]. However, sampling and detection errors can be reduced when the Pap test screening is repeated frequently. If the sensitivity is 87%, the probability of a false-negative rate should theoretically be 13%; thus, the probability of three consecutive Pap tests being false-negative should be 0.2%. Some studies have re-examined Pap tests that were read as normal in women who were subsequently diagnosed with cervical cancer and reported that 25% or less Pap tests were misread, and another 25% may have been performed on inadequate samples [8,9,13]. The false-negative results for precancerous lesions and cancer in the cervix are potentially preventable by a refinement of its interpretations and sampling technique. Because repeating Pap tests is in theory, a simple strategy to overcome false-negative results, the importance of regular screening is emphasized in most countries. However, there are no nationwide data that show a cumulative effect of consecutive Pap tests on false-negative results.
In this nationwide cohort study, we aimed to evaluate the cervical cancer risk through repeated normal Pap tests. We estimated and compared the cumulative incidence rates of cervical cancer diagnosed within 1 year of the last normal Pap test using a nationwide database in groups stratified by the number of consecutive normal Pap tests. We also evaluated the 5-year overall survival rates in women with cervical cancer in each group.
Korean screening guidelines recommended a Pap test of asymptomatic women aged over 20 with either a Pap smear or liquid-based cytology every 3 years (recommendation A) [14]; however, the National Cancer Screening Program (NCSP) freely provides the Pap tests every 2 years to women aged 20 or older. The target population of cervical cancer screening was women aged 30 or older in 2015, but it was expanded to include women over 20 years old in 2016.
The study population is shown in Table 1. In this study, women who had a normal Pap test once in 2011–2012 were assigned to the ‘N1 group’; the N1 group did not receive a Pap test between 2007–2010. Women who had two consecutive normal Pap tests between 2009–2012 and did not undergo a Pap test in 2007–2008 were assigned to the ‘N2 group’. Women who had three consecutive normal Pap tests in 2007–2012 were assigned to ‘N3 group’. Women who did not receive a Pap test in the period 2007–2012 were assigned to the ‘N0 group’. We obtained the Pap test results of 11,052,116 women aged 30–79 in the NCSP from January 1, 2007 to December 31, 2012. Among 2,740,063 women who underwent the Pap test in 2011–2012 through the NCSP, a total of 2,577,070 women had a normal Pap test in 2011–2012 and did not undergo a Pap test between 2007 and 2010 (N1 group). A total of 1,352,226 (N2 group) and 942,156 (N3 group) women had two or three normal Pap tests, respectively, including those of 2011–2012 with 2-year intervals. A total of 6,160,664 women (N0 group) did not receive a Pap test during the 2007–2012 period. The Pap test results were classified by the pathological findings with the 2001 Bethesda system [15]. “Negative for intraepithelial lesion or malignancy” including “organisms” and “other non-neoplastic findings (reactive cellular changes associated with inflammation, intrauterine contraceptive device, atrophy) were considered as normal results.
The operational definition of the incidence of cervical cancer is the first detection of cervical cancer. That is, we measured this incidence based on the codes used in the health insurance claim data set, which employs the Korean Standard Classification of Diseases (C53: malignant neoplasm of the cervix; V193: special case registration for cancer). The date of first treatment was defined as the detection date, and the absence of such treatment was considered to reflect no cancer. Special case registration ensures that the economic burden imposed on patients diagnosed with cerebrovascular and heart diseases, cancer, intractable diseases, or severe burns is decreased by reducing the copayment for treatment by the National Health Insurance Service (NHIS) for 5 years. This system was introduced in July 2001 with a copayment of 20%, and it decreased to 10% in 2005 and to 5% in 2009. Malignant neoplasm of the cervix is included in the criteria for special case registration.
To compare the occurrence of cervical cancer diagnosed within 1 year of the last Pap tests in the N0, N1, N2, and N3 groups, we evaluated the cumulative incidence rates of cervical cancer in each group. The cumulative incidence rates were calculated using the total cancer cases for the 1 year after the last Pap test and the study population of groups and was denoted as case number per 105. For the N0 group, we counted the cases from June 1, 2011 or June 1, 2012 to July 30, 2012 or July 30, 2013, respectively. The cumulative incidence rates were estimated in each age group, and grouped by age as follows: 30–39, 40–49, 50–59, 60–69, and 70–79 years old. Using the cumulative incidence rate, we calculated the relative risk (RR) with a 95% confidence interval (CI). The 5-year survival rate of cancer patients is the most commonly used statistic to reflect improvements in the strategy against cancer [16]. We compared the 5-year overall survival rates in women with cervical cancer in each group.
All analyses were performed using SAS software ver. 9.4 (SAS Institute Inc., Cary, NC) and R ver. 3.3.2 (R Foundation, Vienna, Austria). p-values < 0.05 were considered to indicate statistical significance.
The cumulative incidence rates of cervical cancer diagnosed within 1 year of the last normal Pap test in each group are presented in Table 2. The cumulative incidence rate of cervical cancer was 58.9 per 105 (3,626/6,160,664) in the N0 group, 24.6 per 105 (635/2,577,070) in the N1 group, 20.3 per 105 (275/1,352,226) in the N2 group, and 14.2 per 105 (137/962,156) in the N3 group. The incidence rate was highest in women aged 60–69 (70.4 per 105) and the lowest in women aged 30–39 (45.5 per 105) in the N0 group. However, the age groups with the highest and lowest incidences were different between the groups. In the N3 group, the incidence rate was highest in women aged 60–69 (17.6 per 105), and the lowest in women aged 70–79 (5.1 per 105).
Compared to women with a normal Pap test in the year prior to diagnosis, the risk of cancer was more than twice that of women in the N0 group who did not undergo regular Pap tests (RR, 2.389; 95% CI, 2.196 to 2.599; p < 0.001) (Table 3). As the number of consecutive normal Pap tests increased, the cervical cancer risk in the year after the last normal Pap test gradually decreased. Regardless of age, the cancer risk decreased by 17% (RR, 0.825; 95% CI, 0.716 to 0.951; p=0.008) and 42% (RR, 0.578; 95% CI, 0.480 to 0.695; p < 0.001) in the N2 and N3 group, respectively, compared to those of the N1 group. The cumulative effect of three consecutive normal Pap tests was most marked in women aged 70–79 (RR, 0.127; 95% CI, 0.031 to 0.525; p=0.004).
The 5-year overall survival rates in women with cervical cancer diagnosed within 1 year of the last normal Pap test are presented in Fig. 1. The 5-year overall survival rate of cervical cancer was 70.2% in the N0 group and 81.3% in the N1 group (p < 0.001). The 5-year overall survival rates were 80.0% in the N2 group and 79.6% in the N3 group. Neither the difference in the 5-year survival rates between the N1 and N2 groups (RR, 1.068; 95% CI, 0.752 to 1.514; p=0.715), nor between the N1 and N3 groups (RR, 1.091; 95% CI, 0.695 to 1.712; p=0.706) were statistically significant.
A total of 11,052,116 women were included in the analysis for the cumulative incidence rates and 5-year overall survival rates of cervical cancer which occurred within 1 year of the last normal Pap test. Increasing the number of consecutive normal Pap tests clearly decreases the short-term incidence of cervical cancer. Until now, there have only been a few reports on the incidence of cervical cancer in women with recent normal Pap tests. Rozemeijer et al. [17] reported on the cervical cancer incidence after normal Pap tests by different test methods in the Netherlands. In this study, the 6-year cumulative incidence in women with a normal Pap test by a conventional method was approximately 35 per 105. The authors focused on the sensitivity for progressive cervical intraepithelial neoplasia (CIN) between the different types of liquid-based cytology tests and conventional cytology, and not on consecutive normal Pap tests. In 2003, Sawaya et al. [18] reported that the lifetime prevalence of biopsy-proven high-grade CIN was estimated as 0.019% and the risk of cancer was estimated to be no more than 3 in 105 in women who had three or more consecutive normal Pap tests by simulation using the Markov model. They provided reassurance to women and their healthcare providers that extending the screening interval to 3 years after three or more consecutive normal Pap tests was a safe option.
Cervical cancer that occurs after the last normal Pap test and before the next screening schedule could be considered as screening failure. Misinterpretations and improper testing could be the reason of the shortly-diagnosed cervical cancer both. Several researchers have reported that normal Pap tests performed within 2 years before the cancer diagnosis with misinterpretations could result in delayed diagnosis of precancerous lesions and cancer. After scrutinizing women with cervical cancer in the central cancer registry, Stenkvist and Soderstrom [19] suggested that the true annual incidence of cervical cancer could be considerably decreased if the misinterpretations of the Pap tests were cross-nationally corrected and all women with abnormal Pap tests had been properly managed. Philp et al. [20] also reviewed the Pap test specimens within the 2 years before cancer diagnosis and showed that prior Pap test results were normal in 13.0%, low-grade CIN in 8.2%, high-grade CIN in 52.6%, and others in 18.7% in 1,250 women with cervical cancer, while suspicions for cancer were found in only 7.4% of women. Furthermore, the authors demonstrated a high proportion of false-negative results in women with cervical cancer, consistent with previous reports [21–24].
In our study, the 5-year overall survival rates in women diagnosed with cervical cancer within 1 year of the last normal Pap test were not superior in the N3 group (79.6%) to those of the N1 (81.3%) and N2 (80.0%) groups. Contrary to expectation, consecutive normal Pap tests did not improve the survival outcomes as a result of early detection of disease. Although we could not obtain the histology and disease extent of each woman diagnosed with cervical cancer, we expect that women with cervical cancer who have recent repeated normal Pap tests are likely to demonstrate non-squamous histology or cancer located at the endocervical canal. Furthermore, the histologic type can also affect the diagnostic accuracy of the Pap tests, and is most notable in cases with adenocarcinoma histology. Adenocarcinoma cells can often mimic benign endometrial cells, endocervical cells with tubal metaplasia, or reactive endocervical cells [22,23,25]. In the study by Katki et al. [26], the cancers observed among Pap-negative/human papillomavirus (HPV)–positive women in a 5-year follow-up were mostly those of adenocarcinoma histology, more than would be expected based on the overall adenocarcinoma incidence of 28%. Because 46% of adenocarcinomas occurred in women who were Pap-negative, the Pap test was less effective in screening precancerous lesions of adenocarcinoma. As a result, delayed diagnosis of precancerous lesions and cancer can affect the survival outcomes in women with cervical cancer.
Some rapid-onset cancer cases may contribute to the incidence of cervical cancer within 1 year of a normal Pap test. Hildesheim et al. [27] analyzed questionnaire data from women with cervical cancer and suggested that rapid-onset cases are more frequent in women with a younger age and glandular tumors (adenocarcinomas or adenosquamous carcinomas). Some previous studies have shown that glandular tumors of the cervix are more aggressive than squamous tumors and are associated with a poorer prognosis [28,29]. Cancerous lesions are known to occasionally give incorrect Pap test results with blood-stained, scanty, and only normal cells observed. Furthermore, most patients with apparently rapid-onset cancer only had one previous Pap test. Korean Society of Gynecologic Oncology recommends a Pap test of asymptomatic women aged over 20 with every 3 years and the NCSP supports the Pap tests every 2 years now [14]. The results of this study may affect the guidelines to pursue the Pap test every 1–2 years because it is not hard to pursue annual Pap test in the Korean medical status unlike other western countries.
To the best of our knowledge, this study is the first analysis of a nationwide database to evaluate the cumulative effect of consecutive normal Pap tests on cervical cancer risk. We found a significant preventive effect of cervical cancer by consecutive normal Pap tests in this large-scale population. However, there are some limitations in our study, mainly due to its retrospective nature. Firstly, the observed incidence rate of cervical cancer may be influenced by selection bias. Women who are more likely to return for screening may be women with low-risk who are concerned about their health, but may also be women with high-risk who have a history of cervical abnormalities. Furthermore, we do not know whether the included women had other risk factors for precancerous lesions and cervical cancer. Secondly, we have no data on HPV infection in the included women, because the HPV test is not currently provided by the NSCP in Korea. HPV infection status can affect confirmative examinations and follow-up schedules in the study population. Thirdly, there is a lack of clinical information on diagnosed cervical cancer, including stage, histology, and its treatment course. Testing performers (gynaecologists, family medicine doctors, or others) and methods which can affect the accuracy of the test are also unknown. Lastly, the age distribution is not equal between the study groups. The small size of women aged 30–39 and 70–79 in the N2 and N3 group may affect the incidence pattern of cervical cancer by age.
This nationwide cohort study provides an important message that we can overcome the high false-negative rate of Pap tests by regularly repeating screening. We should more actively promote the importance of consecutive normal Pap tests to prevent cervical cancer, not just recent normal Pap tests, to the general population. Furthermore, future studies to improve the diagnosis accuracy of the Pap test, such as Pap/HPV co-testing, should be conducted to avoid delayed cancer diagnosis and improve survival outcomes.
Notes
Ethical Statement
This study was reviewed and approved by the Institutional Review Boards (approval no. X-1906-549-901) of Seoul National University Bundang Hospital, and the written informed consent was waived because all the information was tabulated in anonymized and de-identified fashion. The National Health Insurance Service (NHIS) database was used (approval no. NHIS-2020-1-059).
ACKNOWLEDGMENTS
This study was supported by grants from the Seoul National University Bundang Hospital (No. 02-2017-023).
References
1. Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018; 68:394–424.

2. Arbyn M, Raifu AO, Weiderpass E, Bray F, Anttila A. Trends of cervical cancer mortality in the member states of the European Union. Eur J Cancer. 2009; 45:2640–8.

3. Wingo PA, Cardinez CJ, Landis SH, Greenlee RT, Ries LA, Anderson RN, et al. Long-term trends in cancer mortality in the United States, 1930–1998. Cancer. 2003; 97:3133–275.

4. Lim MC, Won YJ, Ko MJ, Kim M, Shim SH, Suh DH, et al. Incidence of cervical, endometrial, and ovarian cancer in Korea during 1999–2015. J Gynecol Oncol. 2019; 30:e38.

5. Papanicolaou GN, Traut HF. The diagnostic value of vaginal smears in carcinoma of the uterus 1941. Arch Pathol Lab Med. 1997; 121:211–24.
6. Kong TW, Kim M, Kim YH, Kim YB, Kim J, Kim JW, et al. High-risk human papillomavirus testing as a primary screening for cervical cancer: position statement by the Korean Society of Obstetrics and Gynecology and the Korean Society of Gynecologic Oncology. J Gynecol Oncol. 2020; 31:e31.

7. US Preventive Services Task Force. Curry SJ, Krist AH, Owens DK, Barry MJ, Caughey AB, et al. Screening for cervical cancer: US Preventive Services Task Force recommendation statement. JAMA. 2018; 320:674–86.
8. Walker EM, Hare MJ, Cooper P. A retrospective review of cervical cytology in women developing invasive squamous cell carcinoma. Br J Obstet Gynaecol. 1983; 90:1087–91.

9. Janerich DT, Hadjimichael O, Schwartz PE, Lowell DM, Meigs JW, Merino MJ, et al. The screening histories of women with invasive cervical cancer, Connecticut. Am J Public Health. 1995; 85:791–4.

10. Wain GV, Farnsworth A, Hacker NF. The Papanicolaou smear histories of 237 patients with cervical cancer. Med J Aust. 1992; 157:14–6.

11. Fahey MT, Irwig L, Macaskill P. Meta-analysis of Pap test accuracy. Am J Epidemiol. 1995; 141:680–9.

12. Nanda K, McCrory DC, Myers ER, Bastian LA, Hasselblad V, Hickey JD, et al. Accuracy of the Papanicolaou test in screening for and follow-up of cervical cytologic abnormalities: a systematic review. Ann Intern Med. 2000; 132:810–9.

13. Rylander E. Negative smears in women developing invasive cervical cancer. Acta Obstet Gynecol Scand. 1977; 56:115–8.

14. Min KJ, Lee YJ, Suh M, Yoo CW, Lim MC, Choi J, et al. The Korean guideline for cervical cancer screening. J Gynecol Oncol. 2015; 26:232–9.

15. Solomon D, Davey D, Kurman R, Moriarty A, O’Connor D, Prey M, et al. The 2001 Bethesda system: terminology for reporting results of cervical cytology. JAMA. 2002; 287:2114–9.

16. Maruvka YE, Tang M, Michor F. On the validity of using increases in 5-year survival rates to measure success in the fight against cancer. PLoS One. 2014; 9:e83100.

17. Rozemeijer K, Naber SK, Penning C, Overbeek LI, Looman CW, de Kok IM, et al. Cervical cancer incidence after normal cytological sample in routine screening using SurePath, ThinPrep, and conventional cytology: population based study. BMJ. 2017; 356:j504.

18. Sawaya GF, McConnell KJ, Kulasingam SL, Lawson HW, Kerlikowske K, Melnikow J, et al. Risk of cervical cancer associated with extending the interval between cervical-cancer screenings. N Engl J Med. 2003; 349:1501–9.

19. Stenkvist B, Soderstrom J. Reasons for cervical cancer despite extensive screening. J Med Screen. 1996; 3:204–7.

20. Philp L, Jembere N, Wang L, Gao J, Maguire B, Kupets R. Pap tests in the diagnosis of cervical cancer: help or hinder? Gynecol Oncol. 2018; 150:61–6.

21. DeMay RM. Cytopathology of false negatives preceding cervical carcinoma. Am J Obstet Gynecol. 1996; 175:1110–3.

22. Zhao L, Wentzensen N, Zhang RR, Dunn ST, Gold MA, Wang SS, et al. Factors associated with reduced accuracy in Papanicolaou tests for patients with invasive cervical cancer. Cancer Cytopathol. 2014; 122:694–701.

23. Krane JF, Granter SR, Trask CE, Hogan CL, Lee KR. Papanicolaou smear sensitivity for the detection of adenocarcinoma of the cervix: a study of 49 cases. Cancer. 2001; 93:8–15.
24. Coldman A, Phillips N, Kan L, Matisic J, Benedet L, Towers L. Risk of invasive cervical cancer after three consecutive negative Pap smears. J Med Screen. 2003; 10:196–200.

25. Etherington IJ, Luesley DM. Adenocarcinoma in situ of the cervix-controversies in diagnosis and treatment. J Low Genit Tract Dis. 2001; 5:94–8.

26. Katki HA, Schiffman M, Castle PE, Fetterman B, Poitras NE, Lorey T, et al. Five-year risks of CIN 3+ and cervical cancer among women who test Pap-negative but are HPV-positive. J Low Genit Tract Dis. 2013; 17(5 Suppl 1):S56–63.
27. Hildesheim A, Hadjimichael O, Schwartz PE, Wheeler CM, Barnes W, Lowell DM, et al. Risk factors for rapid-onset cervical cancer. Am J Obstet Gynecol. 1999; 180:571–7.

Fig. 1
Five-year survival rates of cervical cancer diagnosed within 1 year of the last normal Pap test in each study group.
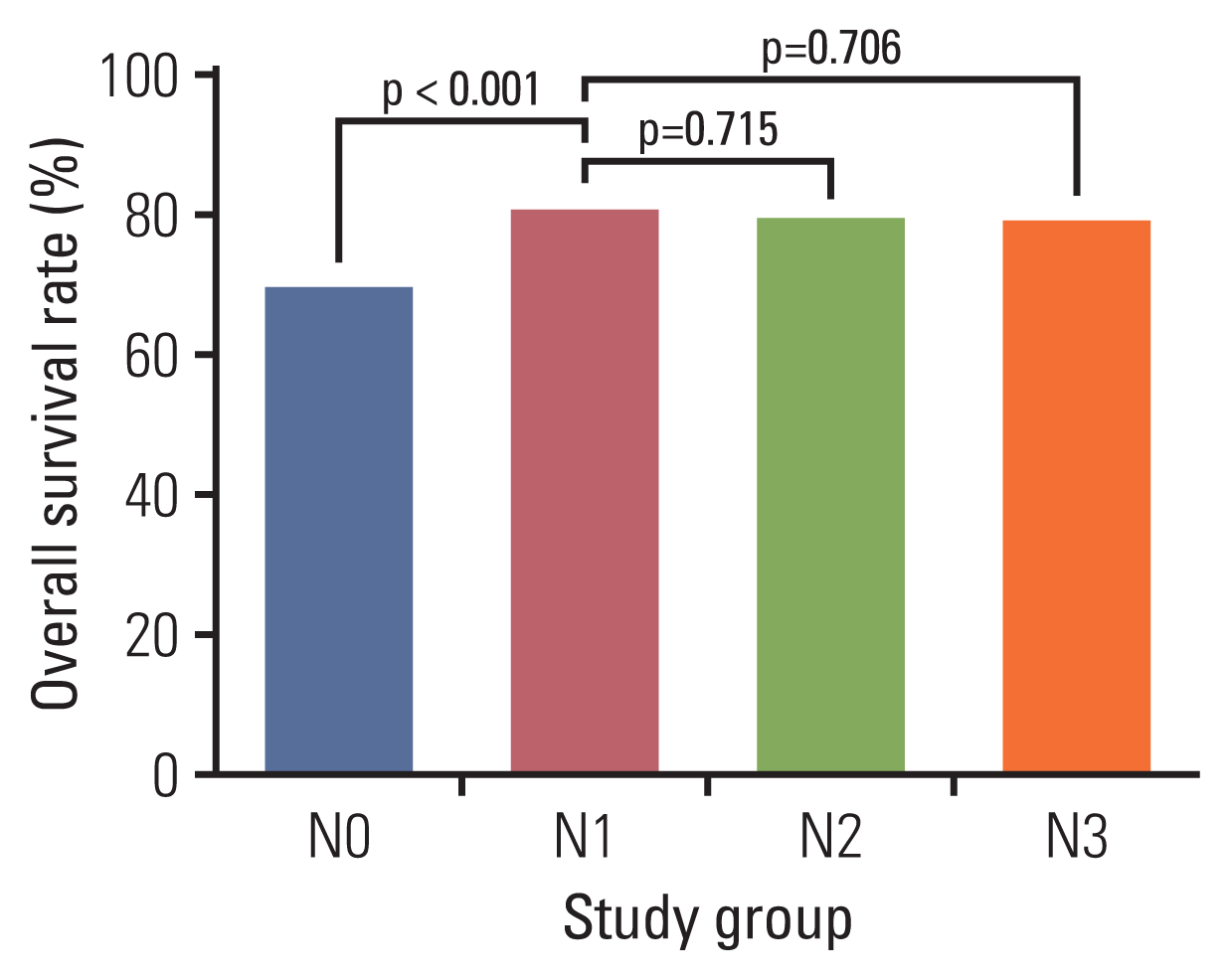
Table 1
Study population
Table 2
Cumulative incidences of cervical cancer diagnosed within 1 year after the last normal Pap test
| Age (yr) | Groups by consecutive number of normal Pap tests | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
|
||||||||||||
| N0 | N1 | N2 | N3 | |||||||||
|
|
|
|
|
|||||||||
| No.a) | Case | Cumulative incidenceb) | No.a) | Case | Cumulative incidenceb) | No.a) | Case | Cumulative incidenceb) | No.a) | Case | Cumulative incidenceb) | |
| 30–39 | 2,287,769 | 1,041 | 45.5 | 1,044,400 | 208 | 19.9 | 311,630 | 44 | 14.1 | 45,539 | 3 | 6.6 |
|
|
||||||||||||
| 40–49 | 1,480,771 | 956 | 64.6 | 727,946 | 194 | 26.7 | 462,682 | 123 | 26.6 | 323,046 | 49 | 15.2 |
|
|
||||||||||||
| 50–59 | 879,899 | 593 | 67.4 | 465,519 | 114 | 24.5 | 352,816 | 67 | 19.0 | 344,100 | 46 | 13.4 |
|
|
||||||||||||
| 60–69 | 715,530 | 504 | 70.4 | 242,245 | 80 | 33.0 | 179,648 | 32 | 17.8 | 210,191 | 37 | 17.6 |
|
|
||||||||||||
| 70–79 | 796,695 | 532 | 66.7 | 97,060 | 39 | 40.2 | 45,450 | 9 | 19.8 | 39,280 | 2 | 5.1 |
|
|
||||||||||||
| All | 6,160,664 | 3,626 | 58.9 | 2,577,070 | 635 | 24.6 | 1,352,226 | 275 | 20.3 | 962,156 | 137 | 14.2 |
Table 3
Relative risk for cervical cancer incidences within 1 year of the last normal Pap test




 PDF
PDF Citation
Citation Print
Print


 XML Download
XML Download