This article has been
cited by other articles in ScienceCentral.
Abstract
Background
For certain ocular movement abnormalities, the exact neuroanatomical localization of the causative lesion is still not defined. Oculomotor apraxia, apraxia of eye opening and closing, and motor impersistence are rarely reported in acute stroke, particularly following venous stroke.
Case Report
A 34-year-old man presented with headache, vomiting, focal seizures with bilateral tonic-clonic movements, and altered sensorium. Magnetic resonance imaging revealed bilateral frontal and left parietal hemorrhagic infarcts, and contrast venography revealed superior sagittal sinus thrombosis. The patient received anticoagulant treatment with antiepileptics. On re-examination on day 3, the patient had a rare combination of apraxia of eyelid closure, motor impersistence, and oculomotor apraxia. By Day 10 of admission, all oculomotor abnormalities had subsided.
Conclusion
To the best of our knowledge, this is the first report of the combination of oculomotor apraxia and apraxia of eyelid closure with motor impersistence in a patient with cerebral venous sinus thrombosis.
Go to :

Keywords: Sinus thrombosis, Apraxia, Frontal lobe
INTRODUCTION
Various eyelid and eye movement abnormalities have been attributed to different central nervous system lesions. Apraxia of eyelid closure (AEC), which is less common than apraxia of eyelid opening, is reported to occur in progressive supranuclear palsy, Creutzfeldt-Jakob disease, Huntington disease, amyotrophic lateral sclerosis, and acquired frontal and parietal lobe diseases such as stroke [
1]. We report a case of cerebral venous sinus thrombosis with a rare combination of oculomotor apraxia (OMA) and AEC with motor impersistence (MI).
Go to :

CASE REPORT
A 34-year-old right-handed man was admitted with a history of acute onset severe holocranial headache, multiple episodes of vomiting, and focal seizures with bilateral tonic-clonic movements followed by altered sensorium for the past 4 days. There was no preceding history of fever, head injury, or chronic drug intake. The patient had no previous comorbidities. There was no significant family history for any similar illnesses.
On examination, the patient’s Glasgow coma scale score was E2V1M4, body temperature 39°C, blood pressure 156/100 mmHg, and respiratory rate 18 per minute with normal oxygen saturation. His pupils were symmetrical and normally reactive to light without papilledema. There were decreased movements of all four limbs with bilateral extensor plantar responses. The remaining findings of the systemic examination were within normal limits.
His complete blood count, blood sugar levels, renal and liver function tests, chest radiography, and electrocardiography results were within normal limits. His D-dimer was 1,146 ng/mL (normal <255 ng/mL). Magnetic resonance imaging (MRI) of the brain revealed heterogeneous hyperintensity in the bilateral frontal and left parietal lobes on T2-weighted/fluid-attenuated inversion recovery images with blooming in susceptibility-weighted images; magnetic resonance venography revealed thrombosis of the anterior two-thirds of the superior sagittal sinus (
Fig. 1). The patient was managed conservatively. His seizures were controlled with levetiracetam (1 g twice daily) and lacosamide (100 mg twice daily). He received low molecular weight heparin (100 U/kg) subcutaneous twice daily. Thrombophilia testing was positive for lupus anticoagulant. His serum homocysteine was 59.1 µmol/L (normal <12 µmol/L) and vitamin B12 was 121 pg/mL (normal >200 pg/mL). He was prescribed cyanocobalamin 1,000 µg, thiamine 100 mg, and pyridoxine 100 mg in combination daily for 7 days and then weekly. On day 3 of admission, his Glasgow coma scale score improved to E4V5M6 without any seizure recurrence. On re-examination, he could not comply with commands regarding eye closure, although reflex eye blinking was normal. He was fully conscious, alert, and cooperative, and followed commands such as mouth opening, tongue protrusion, and chewing, including complex multi-step commands. His visual acuity was 6/6 in both eyes, and there were no field defects. He was unable to maintain eyelid closure for more than a fraction of the second, though reflex blinking to visual, auditory, and corneal stimulation was normal, suggestive of AEC with MI. While sleeping, his eyes remained completely closed. In addition, his gaze was restricted in both horizontal and vertical directions, although the doll’s eye sign, vestibulo-ocular reflex, compensatory head thrust, and intermittent reflexive saccadic movements in all directions in response to visual stimuli were present, suggesting OMA (
Supplementary Video 1). No brainstem lesions were observed on the brain MRI (
Fig. 2). Over a period of 1 week, the eyelid and oculomotor movement abnormalities resolved completely.
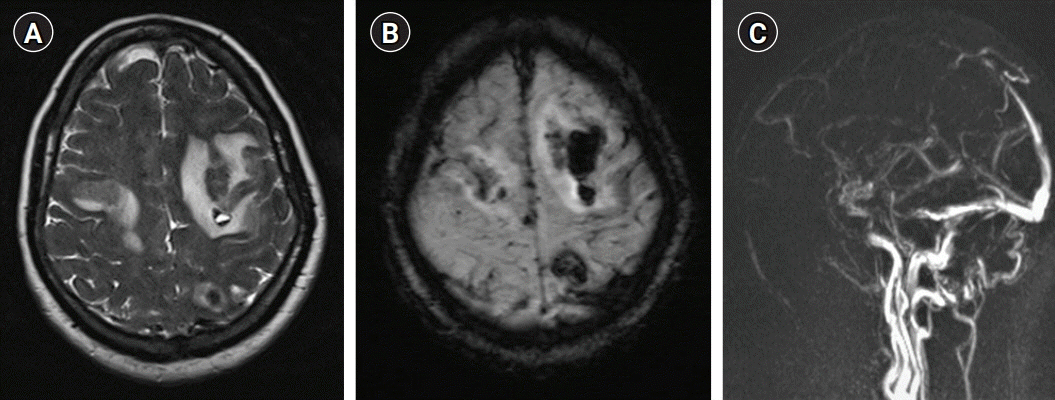 | Fig. 1.Magnetic resonance imaging of the patient showing bilateral frontal and left parietal hyperintensity on T2-weighted images (A), blooming at the same sites on susceptibility-weighted images (B) suggestive of hemorrhagic infarct and thrombosis of the anterior two-third of the superior sagittal sinus (C) on contrast venography. 
|
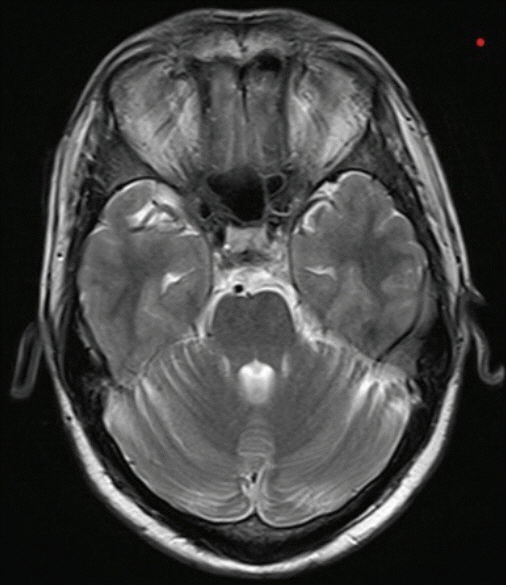 | Fig. 2.Magnetic resonance imaging of the patient showing normal parenchyma at the pontine level. 
|
Go to :

DISCUSSION
The reported patient presented with acute-onset headache, focal with bilateral tonic clonic seizures, and altered sensorium. MRI brain revealed bilateral frontal and left parietal lesions with superior sagittal sinus thrombosis. Cranial nerve examination revealed a rare combination of OMA and AEC with MI.
The inability to perform voluntary eye closure on command while retaining spontaneous blinking was first described by Roth in 1901 as a “pseudobulbar paralysis” phenomenon and later coined as “apraxia of eyelid closure” by Lewandowsky in 1907 [
2]. Which hemisphere is dominant in supranuclear control of eyelid movements is a matter of debate. In a systematic review, Nicoletti et al. described that all 15 patients with unilateral AEC had contralateral lesions, that is, a right hemisphere lesion, whereas a similar hemispheric lesion was observed in 90% (17/19) of patients with bilateral AEC [
3]. They also mentioned that, in contrast to apraxia of eyelid opening, where subcortical structures are commonly affected, the frontoparietal cortex is predominantly involved in AEC. Functional MRI of the brain has explored the role of the frontal eye field (FEF), supplementary eye field (SEF), and posterior parietal cortex (PPC) in voluntary eye closure. Voluntary bilateral eye closure (blinking) activates the FEF and SEF but not the PPC. In contrast, voluntary unilateral eye closure (winking) activates a frontoparietal network involving the FEF, SEF, and PPC [
4]. In the given case, bilateral AEC was probably due to involvement of the bilateral frontal cortex.
OMA was first described by Cogan [
5] as the inability to generate horizontal saccades voluntarily in children with congenital OMA. Later, this abnormality was described in many other congenital and acquired neurological diseases [
1]. Voluntary and reflexive saccades are generated in the FEF and interparietal sulcus, respectively. While horizontal saccades are initiated by the contralateral FEF and superior colliculus, vertical saccades are associated with activity in the FEFs and superior colliculi bilaterally [
6]. Our patient had impaired horizontal and vertical voluntary saccades due to bilateral FEF involvement.
MI is defined as the inability to sustain a certain position or movement. This symptom was first described by Fisher [
7] in patients with right hemispheric stroke. Patients with frontal or subcortical lesions were significantly more likely to demonstrate MI than those with posterior lesions. Moreover, lesions were more common in the right hemisphere than in the left hemisphere, supporting right hemispheric dominance [
8,
9]. Callosal lesions that disconnect the left hemisphere from right hemisphere inputs have also been associated with impersistence of the right limbs [
10]. Our patient had eyelid and OMA with MI. Radiologically, the lesions were also located in the bilateral frontal and left parietal lobes, which are common sites responsible for such abnormalities.
In conclusion, non-motor signs of the eye have been previously reported in other pathologies involving the frontal and parietal cortices. However, to the best of our knowledge, this is the first case report of this combination of OMA and AEC with MI in a patient with cerebral venous sinus thrombosis.
Go to :






 PDF
PDF Citation
Citation Print
Print





 XML Download
XML Download