Abstract
Purpose
Materials and Methods
Results
Supplementary Information
Notes
Author Contributions
Conceived and designed the analysis: Tang SQ, Tang LL, Mao YP, Xu C, Ma J.
Collected the data: Tang SQ, Tang LL, Mao YP, Li WF, Chen L, Zhang Y, Guo Y.
Contributed data or analysis tools: Tang SQ, Tang LL, Mao YP, Li WF, Chen L.
Performed the analysis: Tang SQ, Tang LL, Mao YP, Zhang Y, Guo Y, Liu Q.
Wrote the paper: Tang SQ, Tang LL, Mao YP.
Review: Sun Y, Xu C, Ma J.
ACKNOWLEDGMENTS
References
Fig. 1

Fig. 2
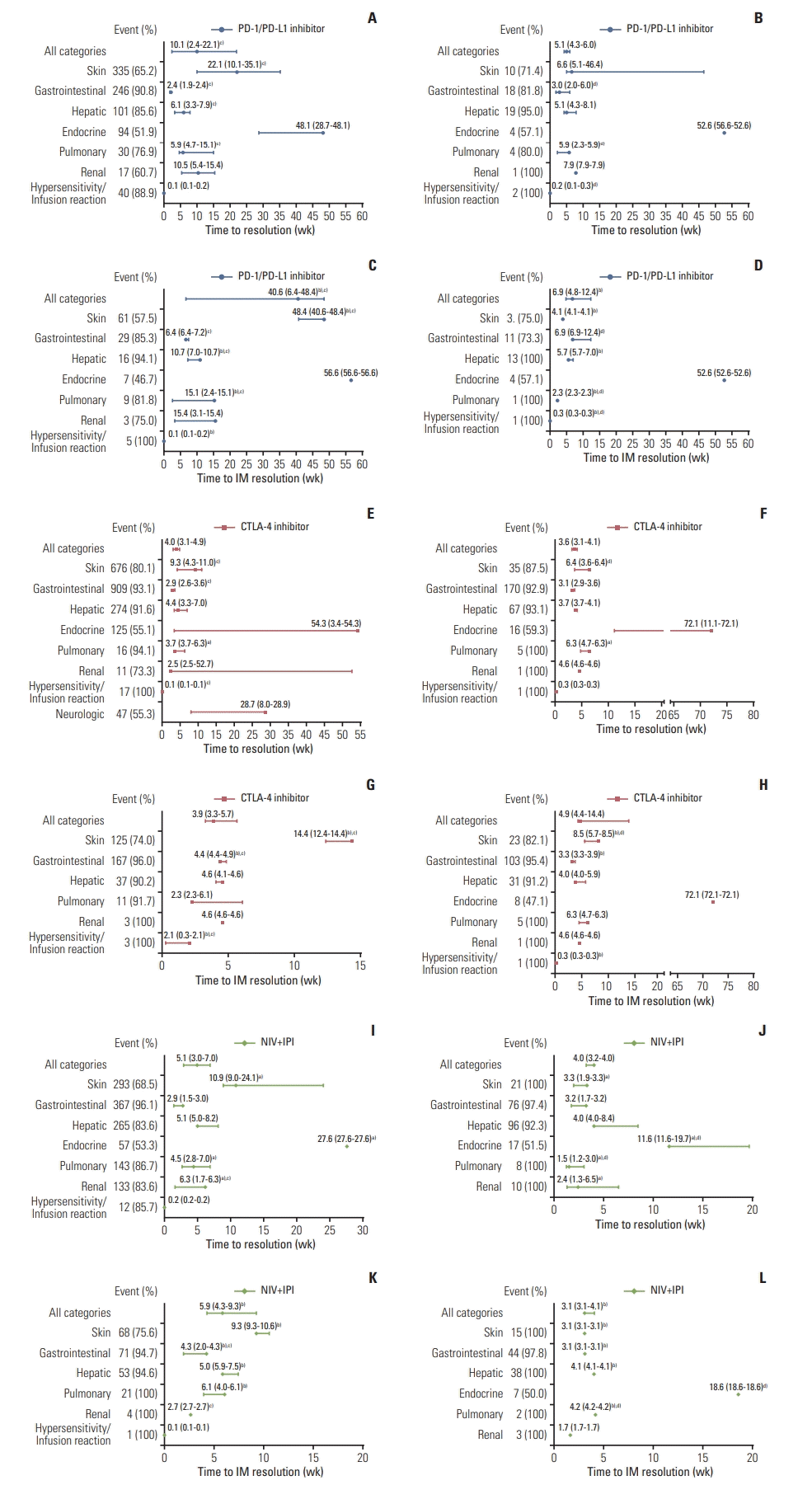
Fig. 3
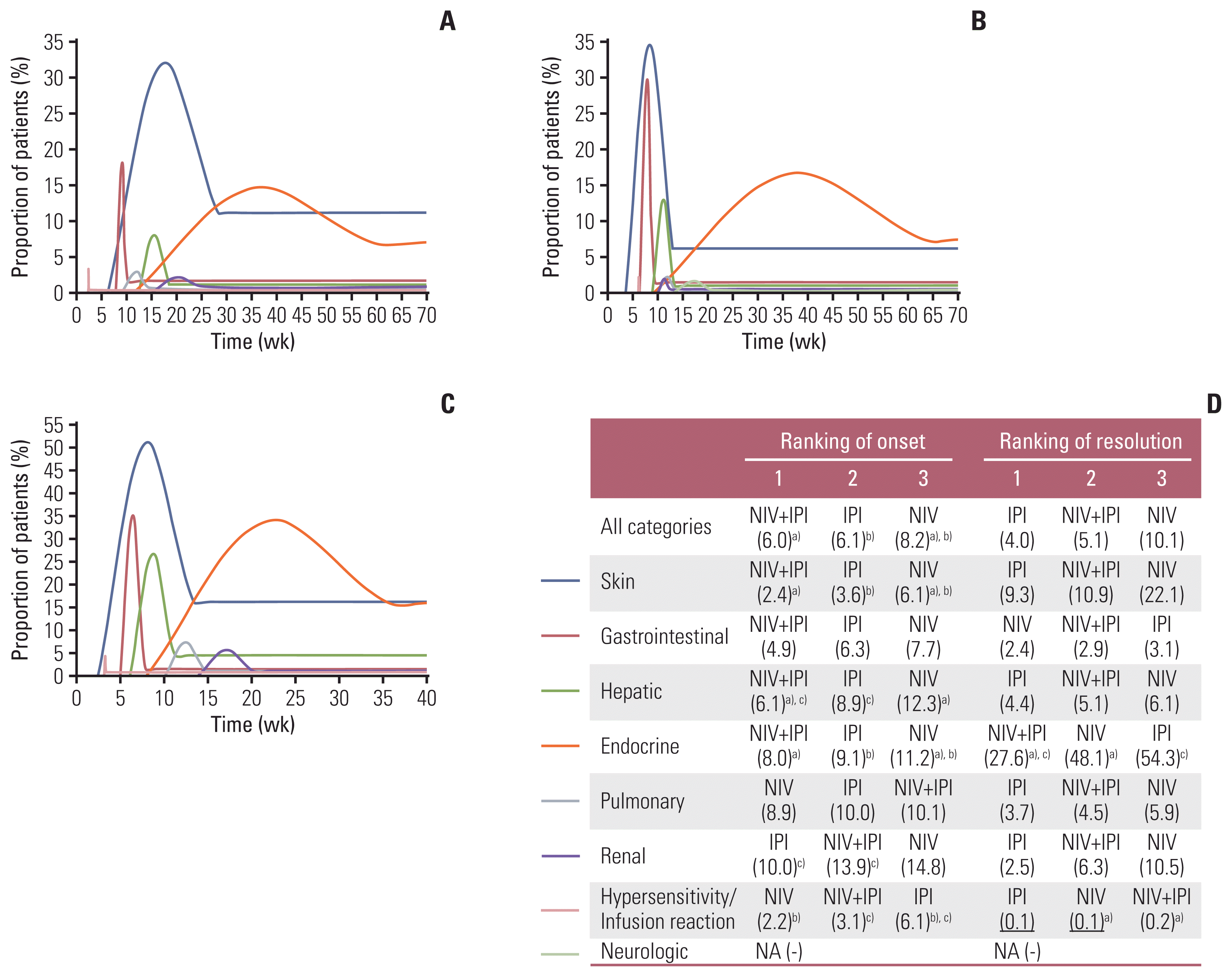
Table 1
| Author, year | Study ID | Region | Cancer | Phase | Total No. | Safety analysis No. | Arm | Treatment | Median follow-up time (mo) | CTCAE version |
|---|---|---|---|---|---|---|---|---|---|---|
| Weber (2013) [21] | MDX010-20 | MN | Melanoma | III | 676 | 403 | 1 | IPI 3 mg/kg Q3W plus gp100 | 21.0 | 3.0 |
| 131 | 2 | IPI 3 mg/kg Q3W | 27.8 | |||||||
| 136 | 3 | Gp 100 | 17.2 | |||||||
| Kwon (2014) [22] | CA184-043 | MN | Prostate cancer | III | 799 | 399 | 1 | IPI 10 mg/kg Q3W plus bone-directed radiotherapy | 9.9 | 3.0 |
| 400 | 2 | Placebo plus bone-directed radiotherapy | 9.3 | |||||||
| Brahmer (2015) [3] | CheckMate 017 | MN | Lung cancer | III | 272 | 131 | 1 | NIV 3 mg/kg Q2W | Min 11.0 | 4.0 |
| 129 | 2 | DOC 75 mg/m2 Q3W | Min 11.0 | |||||||
| Borghaei (2015) [23] | CheckMate 057 | MN | Lung cancer | III | 582 | 287 | 1 | NIV 3 mg/kg Q2W | Min 13.2 | 4.0 |
| 268 | 2 | DOC 75 mg/m2 Q3W | Min 13.2 | |||||||
| Reck (2016) [25] | CA184-156 | MN | Lung cancer | III | 954 | 478 | 1 | IPI 10 mg/kg Q3W, ETO, and DDP or CBP | 10.5 | 3.0 |
| 476 | 2 | ETO and DDP or CBP | 10.2 | |||||||
| Eggermont (2016) [24] | EORTC 18071 | MN | Melanoma | III | 951 | 471 | 1 | IPI 10 mg/kg Q3W | 63.6 | 3.0 |
| 476 | 2 | Placebo Q3W | 64.8 | |||||||
| Weber (2017) [27] | CheckMate 238 | MN | Melanoma | III | 905 | 452 | 1 | NIV 3 mg/kg Q2W | 19.5 | 4.0 |
| 453 | 2 | IPI 10 mg/kg Q3W | 19.5 | |||||||
| Ascierto (2017) [26] | CA184-169 | MN | Melanoma | III | 727 | 364 | 1 | IPI 10 mg/kg Q3W | 14.5 | 3.0 |
| 362 | 2 | IPI 3 mg/kg Q3W | 11.2 | |||||||
| Larkin (2017) [28] | CheckMate 037 | MN | Melanoma | II | 405 | 268 | 1 | NIV 3 mg/kg Q2W | 24.0 | 4.0 |
| 102 | 2 | ICC (DTIC 1,000 mg/m2 Q3W or CBP AUC=6 and PTX 175 mg/m2 Q3W) | 24.0 | |||||||
| Govindan (2017) [29] | CA184-104 | MN | Lung cancer | III | 749 | 388 | 1 | IPI 10 mg/kg Q3W, PTX and CBP | 12.5 | 3.0 |
| 361 | 2 | PTX and CBP | 11.8 | |||||||
| Horn (2017) [30] | CheckMate 017 | MN | Lung cancer | III | 854 | 418 | 1 | NIV 3 mg/kg Q2W | Min 24.0 | 4.0 |
| CheckMate 057 | MN | Lung cancer | III | 397 | 2 | DOC 75 mg/m2 Q3W | Min 24.0 | |||
| Armand (2018) [31] | CheckMate 205 | MN | Hodgkin lymphoma | II | 243 | 243 | 1 | NIV 3 mg/kg Q2W | 18.0 | 4.0 |
| Kelly (2018) [32] | JAVELIN Solid Tumor | MN | Solid tumors | Ia) | 1,650 | 1,650 | 1 | AVE 10 mg/kg Q2W | Min 3.0 | 4.0 |
| JAVELIN Merkel 200 | MN | Merkel cell carcinoma | II | 88 | 88 | 1 | AVE 10 mg/kg Q2W | Min 9.0 | 4.0 | |
| Larkin (2019) [34] | CheckMate 067 | MN | Melanoma | III | 945 | 313 | 1 | NIV 1 mg/kg plus IPI 3 mg/kg Q3W, followed by NIV 3 mg/kg Q2W | Min 60.0 | 4.0 |
| 313 | 2 | NIV 3 mg/kg Q2W | 36.9 | |||||||
| 311 | 3 | IPI 3 mg/kg Q3W | 19.9 | |||||||
| Geoerger (2019) [35] | Keynote 051 | MN | Advanced pediatric cancer | I–II | 154 | 154 | 1 | PEM 2 mg/kg Q3W | 8.6 | 4.0 |
| Fradet (2019) [33] | Keynote 045 | MN | Urothelial carcinoma | III | 542 | 270 | 1 | PEM 200 mg Q3W | 27.7 | 4.0 |
| 272 | 2 | PTX 175 mg/m2 Q3W, DOC 75 mg/m2 Q3W, or VIN 320 mg/m2 Q3W | 27.7 | |||||||
| Tomita (2020) [41] | CheckMate 214 | Japan | Renal cell carcinoma | III | 72 | 38 | 1 | NIV 3 mg/kg plus IPI 1 mg/kg Q3W, followed by NIV 3 mg/kg Q2W | 32.4 | 4.0 |
| 34 | 2 | SUN 50 mg QD for 4 weeks Q6W | 32.4 | |||||||
| Lebbe (2019) [38] | CheckMate 511 | MN | Melanoma | IIIB/IV | 358 | 180 | 1 | NIV 3 mg/kg plus IPI 1 mg/kg Q3W, followed by NIV 480 mg Q4W | 18.8 | 4.0 |
| 178 | 2 | NIV 1 mg/kg plus IPI 3 mg/kg Q3W, followed by NIV 480 mg Q4W | 18.6 | |||||||
| Sharma (2020) [40] | CheckMate 032 | MN | Urothelial carcinoma | I/II | 274 | 78 | 1 | NIV 3 mg/kg Q2W | Min 37.7 | 4.0 |
| 104 | 2 | NIV 3 mg/kg plus IPI 1 mg/kg Q3W, followed by NIV 3 mg/kg Q2W | Min 38.8 | |||||||
| 92 | 3 | NIV 1 mg/kg plus IPI 3 mg/kg Q3W, followed by NIV 3 mg/kg Q2W | Min 7.9 | |||||||
| Morse (2019) [39] | CheckMate 142 | MN | Colorectal cancer | II | 119 | 119 | 1 | NIV 3 mg/kg plus IPI 1 mg/kg Q3W, followed by NIV 3 mg/kg Q2W | 13.4 | 4.0 |
| Carneiro (2019) [36] | NA | MN | Adrenocortical carcinoma | II | 10 | 10 | 1 | NIV 240 mg Q2W | 4.5 | 4.0 |
| Horinouchi (2019) [37] | ONO 4538 05 | Japan | Lung cancer | II | 35 | 35 | 1 | NIV 3 mg/kg Q2W | 36.0 | 4.0 |
| ONO 4538 06 | Japan | Lung cancer | II | 76 | 76 | 1 | NIV 3 mg/kg Q2W | 36.0 | 4.0 |
AUC, area under the curve; AVE, avelumab; CBP, carboplatin; CTCAE, Common Terminology Criteria for Adverse Events; DDP, cisplatin; DOC, docetaxel; DTIC, dacarbazine; ETO, etoposide; ICC, investigator’s choice chemotherapy; IPI, ipilimumab; MN, multinational; NA, not available; NIV, nivolumab; PEM, pembrolizumab; PTX, paclitaxel; Q2W, every 2 weeks; Q3W, every 3 weeks; Q6W, every 6 weeks; SUN, sunitinib; VIN, vinflunine.
a) The study of Kelly et al. [32] reported pooled results of phase I and phase II clinical trials with a large sample size (n=1,738); thus, the phase I trial was also included in the analysis.
Table 2
| IPI-3 | IPI-10 | NIV-1+IPI-3 | NIV-3+IPI-1 | |
|---|---|---|---|---|
| All categories | ||||
| No. of patients with irAE | 606 (13.5) | 1,565 (20.3) | 921 (29.9) | 402 (19.9) |
| Time to onset (wk) | 5.1 (3.6–7.1) | 6.3 (4.1–8.9) | 4.9 (2.4–6.1) | 6.1 (5.2–9.0) |
| No. of patients with irAE | 495 (87.3) | 1,252 (85.2) | 663 (79.0) | 607 (82.8) |
| Time to resolution (wk) | 3.6 (2.9–11.0) | 4.4 (3.1–7.0) | 5.1 (2.9–10.9) | 5.0 (1.8–6.3) |
| Skin | ||||
| No. of patients with irAE | 218 (32.4) | 460 (35.7) | 288 (58.7) | 135 (40.1) |
| Time to onset (wk) | 3.6 (3.6–5.1) | 2.6 (2.6–4.1) | 2.1 (2.1–2.4)a) | 5.1 (3.1–5.2)a) |
| No. of patients with resolution | 179 (82.1) | 377 (82.0) | 193 (67.2) | 100 (70.9) |
| Time to resolution (wk) | 11 (5.1–11.0) | 9.3 (3.1–9.3) | 10.9 (10.9–24.1) | 9.0 (9.0–13.1) |
| Gastrointestinal | ||||
| No. of patients with irAE | 231 (34.3) | 567 (44.0) | 207 (42.2) | 84 (24.9) |
| Time to onset (wk) | 7.1 (4.6–7.6) | 6.3 (4.4–7.6) | 4.9 (3.9–4.9) | 6.1 (3.6–9.1) |
| No. of patients with resolution | 218 (94.8) | 539 (95.1) | 197 (95.6) | 170 (96.6) |
| Time to resolution (wk) | 2.9 (2.9–3.6) | 3.1 (2.1–4.0) | 2.9 (2.9–3.0)a) | 1.5 (1.5–2.7)a) |
| Hepatic | ||||
| No. of patients with irAE | 32 (4.8) | 223 (17.3) | 163 (33.2) | 58 (17.2) |
| Time to onset (wk) | 8.9 (6.1–9.0) | 8.9 (8.1–8.9) | 6.0 (6.0–6.1)a) | 9.0 (7.0–10.0)a) |
| No. of patients with resolution | 30 (93.8) | 205 (91.9) | 148 (90.8) | 117 (76.0) |
| Time to resolution (wk) | 4.1 (2.9–4.1) | 4.4 (4.4–7.0) | 5.1 (5.1–6.1) | 5.0 (2.0–8.2) |
| Endocrine | ||||
| No. of patients with irAE | 57 (8.5) | 269 (20.9) | 192 (39.1) | 89 (26.4) |
| Time to onset (wk) | 9.1 (8.9–9.1)b) | 10.2 (8.9–10.2)b) | 8.0 (6.0–8.0) | 6.1 (6.1–12.0) |
| No. of patients with resolution | 14 (70.0) | 93 (53.8) | 57 (53.3) | - |
| Time to resolution (wk) | 3.4 (3.4–3.4)b) | 54.3 (13.9–54.3)b) | 27.6 (27.6–27.6) | NA |
| Pulmonary | ||||
| No. of patients with irAE | 6 (1.9) | 11 (2.4) | 25 (8.0) | 22 (6.5) |
| Time to onset (wk) | 10.1 (10.1–10.1) | 10.0 (10.0–10.0) | 10.1 (10.1–10.1)a) | 15.4 (10.5–16.6)a) |
| No. of patients with resolution | 5 (83.3) | 11 (100) | 29 (96.7) | 114 (84.4) |
| Time to resolution (wk) | 6.3 (6.3–6.3) | 3.7 (3.7–3.7) | 7.0 (3.0–7.0) | 4.5 (2.8–14.6) |
| Renal | ||||
| No. of patients with irAE | 8 (2.6) | 7 (1.5) | 32 (6.5) | 14 (4.2) |
| Time to onset (wk) | 10.0 (10.0–10.0) | 9.7 (9.7–9.7) | 13.9 (8.7–13.9)a) | 15.7 (12.6–36.4)a) |
| No. of patients with resolution | 7 (87.5) | 4 (57.1) | 27 (84.4) | 106 (83.5) |
| Time to resolution (wk) | 2.5 (2.5–2.5) | 52.7 (52.7–52.7) | 2.1 (1.3–2.1) | 6.3 (1.6–6.9) |
| Hypersensitivity/Infusion reaction | ||||
| No. of patients with irAE | 8 (2.6) | 9 (2.0) | 14 (4.5) | - |
| Time to onset (wk) | 4.3 (4.3–4.3) | 6.1 (6.1–6.1) | 3.1 (3.1–3.1) | NA |
| No. of patients with resolution | 8 (100) | 9 (100) | 12 (85.7) | - |
| Time to resolution (wk) | 0.1 (0.1–0.1) | 0.1 (0.1–0.1) | 0.2 (0.2–0.2) | NA |
| Neurologic | ||||
| No. of patients with irAE | 1 (0.3) | 19 (2.3) | - | - |
| Time to onset (wk) | 11.7 (11.7–11.7)b) | 13.1 (10.4–13.1)b) | NA | NA |
| No. of patients with resolution | 1 (100) | 14 (73.7) | - | - |
| Time to resolution (wk) | 0.7 (0.7–0.7) | 8.0 (8.0–11.6) | NA | NA |
Values are presented as number (%) or median (95% confidence interval). ICI, immune checkpoint inhibitor; IPI-1, ipilimumab 1 mg/kg Q3W; IPI-3, ipilimumab 3 mg/kg Q3W; IPI-10, ipilimumab 10 mg/kg Q3W; irAE, immune-related adverse event; NA, not available; NIV-1, nivolumab 1 mg/kg Q3W; NIV-3, nivolumab 3 mg/kg Q3W.
Table 3
| Lung cancer | Melanoma | |
|---|---|---|
| All categories | ||
| No. of patients with irAE | 800 (10.0) | 4,359 (18.3) |
| Time to onset (wk) | 4.7 (4.7–5.7)a) | 6.1 (5.7–7.6)a) |
| No. of patients with irAE | 502 (76.9) | 3,222 (80.5) |
| Time to resolution (wk) | 4.0 (2.7–9.4) | 4.4 (3.4–6.9) |
| Skin | ||
| No. of patients with irAE | 270 (19.4) | 1,496 (40.8) |
| Time to onset (wk) | 4.7 (2.9–5.7) | 4.0 (2.6–5.7) |
| No. of patients with resolution | 178 (76.7) | 1,026 (72.6) |
| Time to resolution (wk) | 9.4 (4.3–10.1) | 10.9 (5.1–22.1) |
| Gastrointestinal | ||
| No. of patients with irAE | 226 (16.2) | 1,294 (35.3) |
| Time to onset (wk) | 4.5 (4.4–22.4) | 6.3 (4.6–7.6) |
| No. of patients with resolution | 186 (86.5) | 1,217 (94.3) |
| Time to resolution (wk) | 2.7 (2.3–2.9) | 2.9 (2.4–3.1) |
| Hepatic | ||
| No. of patients with irAE | 74 (5.3) | 542 (14.8) |
| Time to onset (wk) | 8.0 (2.0–9.0) | 8.9 (6.1–9.0) |
| No. of patients with resolution | 56 (83.6) | 491 (90.6) |
| Time to resolution (wk) | 3.3 (2.0–4.0)a) | 5.1 (4.4–6.1)a) |
| Endocrine | ||
| No. of patients with irAE | 107 (7.7) | 749 (20.4) |
| Time to onset (wk) | 11.2 (8.9–13.3) | 8.9 (8.0–10.2) |
| No. of patients with resolution | 18 (52.9) | 258 (53.6) |
| Time to resolution (wk) | 10.4 (10.4–10.4) | 29.1 (13.9–54.3) |
| Pulmonary | ||
| No. of patients with irAE | 25 (4.7) | 77 (3.1) |
| Time to onset (wk) | 27.9 (4.8–27.9)a) | 10.1 (8.7–10.1)a) |
| No. of patients with resolution | 16 (84.2) | 69 (89.6) |
| Time to resolution (wk) | 5.9 (5.9–5.9) | 6.3 (3.0–7.0) |
| Renal | ||
| No. of patients with irAE | 17 (3.2) | 70 (2.8) |
| Time to onset (wk) | 8.2 (8.2–17.8)a) | 13.9 (9.7–15.7)a) |
| No. of patients with resolution | 6 (54.5) | 54 (77.1) |
| Time to resolution (wk) | 10.5 (10.5–10.5)a) | 2.3 (2.1–10.5)a) |
| Hypersensitivity/Infusion reaction | ||
| No. of patients with irAE | 16 (3.0) | 66 (3.1) |
| Time to onset (wk) | 0.2 (0.2–1.8)a) | 3.3 (2.2–6.1)a) |
| No. of patients with resolution | 10 (100) | 59 (89.4) |
| Time to resolution (wk) | 0.1 (0.1–0.1) | 0.1 (0.1–0.2) |
| Neurologic | ||
| No. of patients with irAE | 65 (7.5) | 20 (1.7) |
| Time to onset (wk) | 4.0 (4.0–7.1)a) | 13.1 (10.4–13.1)a) |
| No. of patients with resolution | 32 (49.2) | 15 (75.0) |
| Time to resolution (wk) | 28.7 (28.7–28.9)a) | 8.0 (0.7–11.6)a) |




 PDF
PDF Citation
Citation Print
Print


 XML Download
XML Download