Abstract
Purpose
Squamous cell carcinomas (SqCC) of the lung often express high levels of thymidylate synthase (TS), which is associated with primary resistance to pemetrexed. We explored the efficacy of pemetrexed in a selected population of patients with lung SqCC with low TS expression.
Materials and Methods
In this single-arm phase II trial, we enrolled 32 previously-treated patients with advanced lung SqCC exhibiting low immunohistochemical staining for TS (i.e., in 10% or less of tumor cells). The primary endpoint was 12-week progression-free survival (PFS) rate.
Results
Of 32 patients, eight patients (25%) had an Eastern Cooperative Oncology Group performance status of 2, and seven patients (22%) had previously received three or more lines of chemotherapy. The disease control rate from pemetrexed treatment was 30%, and no objective response was observed. The 12-week PFS rate was 24.5% (95% confidence interval [CI], 13.0 to 46.1). Median PFS was 1.3 months (95% CI, 1.3 to 2.7), and median overall survival was 11.8 months (95% CI, 8.1 to not applicable). Most of adverse events were grade 1 or 2.
Squamous cell carcinoma (SqCC), the second most common histologic type of non–small cell lung cancer (NSCLC), accounts for 20%–30% of NSCLC cases [1]. Though recent advances in molecular diagnosis and treatment have significantly improved the survival of patients with advanced NSCLC, patients with advanced lung SqCC still have a poor prognosis. Patients with lung SqCC are often diagnosed at older ages and present with multiple comorbidities that make them vulnerable to treatment-induced toxicities [2,3]. Moreover, most therapeutic breakthroughs with survival benefits are not applicable to this histologic type. Lung SqCC is directly related to tobacco smoking exposure and thus has a high mutation rate and complex genomic alterations that complicate efforts to develop effective targeted therapies against it [4,5].
Recently, immune checkpoint inhibitors targeting programmed cell death protein ligand one demonstrated superior efficacy compared with that of cytotoxic chemotherapy in several clinical trials [6–8]. These drugs have thus been successfully incorporated into the treatment of patients with advanced NSCLC, including SqCC. Nevertheless, cytotoxic chemotherapy is still a mainstay of treatment for patients with advanced lung SqCC, either combined with immunotherapy or as a salvage treatment after failure of immunotherapy. Therefore, an unmet need remains for new, less toxic cytotoxic agents to improve the survival of patients with advanced lung SqCC.
Pemetrexed is an antimetabolite that inhibits multiple enzymes in the folate pathway. In three large randomized clinical trials, pemetrexed showed similar efficacy and a favorable safety profile compared with standard treatment arms [9–11]. However, the subgroup analyses in these studies revealed that the efficacy of this drug varied significantly by histologic subtype and it was relatively ineffective against lung SqCC [12,13]. Based on these results, pemetrexed has been established as the standard treatment for patients with advanced non-SqCC of the lung.
Translational studies have been conducted to discover additional predictive biomarkers for pemetrexed efficacy beyond histologic type. Among the candidate biomarkers, thymidylate synthase (TS) has been investigated in many preclinical and clinical studies as a potential determinant of sensitivity to pemetrexed. This enzyme, which is involved in DNA replication and repair, is the primary target of action for pemetrexed [14]. Ozasa et al. [15] reported that upregulation of the gene encoding TS in lung cancer cell lines may lead to pemetrexed resistance. Several retrospective studies showed that TS overexpression was associated with poor response to pemetrexed treatment [16]. A randomized phase II trial of 321 Korean patients with advanced nonsquamous NSCLC found that pemetrexed plus cisplatin had superior efficacy as a first-line treatment compared with gemcitabine plus cisplatin in TS-low patients but not in TS-high patients [17]. In the same context, many researchers reported that pemetrexed is ineffective in SqCC because this tumor type has higher TS expression than other lung cancer types [18,19]. In a Japanese retrospective study, the RNA expression level of TS was two times higher in lung SqCC (n=520) than in lung adenocarcinoma (n=1,352) [19]. We thus designed a phase II study to explore the efficacy and safety of pemetrexed in selected patients with lung SqCC with low TS expression.
This open-label, single-arm phase II trial was conducted at the National Cancer Center in Korea between July 2016 and November 2019 (KCT0003518). Eligible patients had stage IV cytologically or histologically confirmed squamous-cell NSCLC with immunohistochemical staining indicating low TS expression (i.e., in 10%or less of tumor cells) and had progressed during or after at least one platinum-based regimen. Patients were required to be at least 19 years of age and to have had either measurable or assessable lesions according to Response Evaluation Criteria in Solid Tumors (RECIST) ver. 1.1 [20]. Other eligibility requirements included Eastern Cooperative Oncology Group (ECOG) performance status of 0 to 2, adequate bone marrow (white blood cell count ≥ 2,000/mm3, hemoglobin level ≥ 9.0 g/dL, and platelet count ≥ 100,000/mm3), and sufficient renal (serum creatinine level ≤ 1.5 mg/dL or creatinine clearance ≥ 45 mL/min) and hepatic (in the absence of liver metastases, total bilirubin level ≤ 2 times the upper limit of normal [ULN] and serum transaminase ≤ 2.5 times the ULN; in the presence of liver metastases, total bilirubin level ≤ 3 times the ULN and serum transaminase ≤ 5 times the ULN) function.
Diagnosis of SqCC was confirmed histologically by a lung cancer pathologist (G.K.L.) based on hematoxylin and eosin and p63 staining of pathology slides. Expression of TS in paraffin-embedded tumor tissue was evaluated by immunohistochemistry (IHC) analysis with a monoclonal anti-TS antibody (4H4B1, Invitrogen, Carlsbad, CA). Low TS expression was defined as TS expression in 10% or less of the tumor cells, as described previously [17].
Pemetrexed (Pemed-S, Samyang Biopharm, Seongnam, Korea) was given at a dose of 500 mg/m2 on day 1 of a 3-week cycle. All patients received daily oral supplements of 1 mg folate and intramuscular injections of 1 mg vitamin B12 every 9 weeks. Supplementation began 1 week before the initiation of study treatment and continued until 3 weeks after the end of treatment to prevent severe treatment-related myelosuppression and mucositis [21]. Study participants were also prescribed 4 mg dexamethasone twice daily on the day before, the day of, and the day after each dose of pemetrexed. Treatment was continued until disease progression, the occurrence of unacceptable toxic effects, or withdrawal of patient consent. Doses could be reduced or delayed to allow recovery from toxic effects at the discretion of the treating physician.
Tumor responses were assessed every two cycles using computed tomography scans. Designation of complete response, partial response, stable disease, or progressive disease was based on the definitions established by RECIST 1.1 [20]. Toxicity evaluations were based on the National Cancer Institute’s Common Terminology Criteria for Adverse Events ver. 4.0.
The primary endpoint of this study was the progression-free survival (PFS) rate at 12 weeks, which was shown to be strongly predictive of subsequent survival in patients with advanced NSCLC [22]. The 12-week PFS rate was defined as the proportion of patients alive without evidence of disease progression at 12 weeks from the start of the study treatment. To detect an increase in the 12-week PFS rate from 40% to 55% with pemetrexed, a sample size of 28 patients was required with a power of 80% and a one-sided α level of 10%. Assuming a drop-out rate of 10%, an initial sample size of 32 patients was planned for enrollment.
The secondary endpoints of this study were PFS, overall survival (OS), and safety. The PFS was calculated as the time from the date of treatment initiation to the first documented date of disease progression, death, or the last follow-up visit. The OS was calculated as the time from the date of treatment initiation to the date of death, or the last follow-up visit. R ver. 4.0.0 (R Software, R Foundation for Statistical Computing, Vienna, Austria) was used for all analyses.
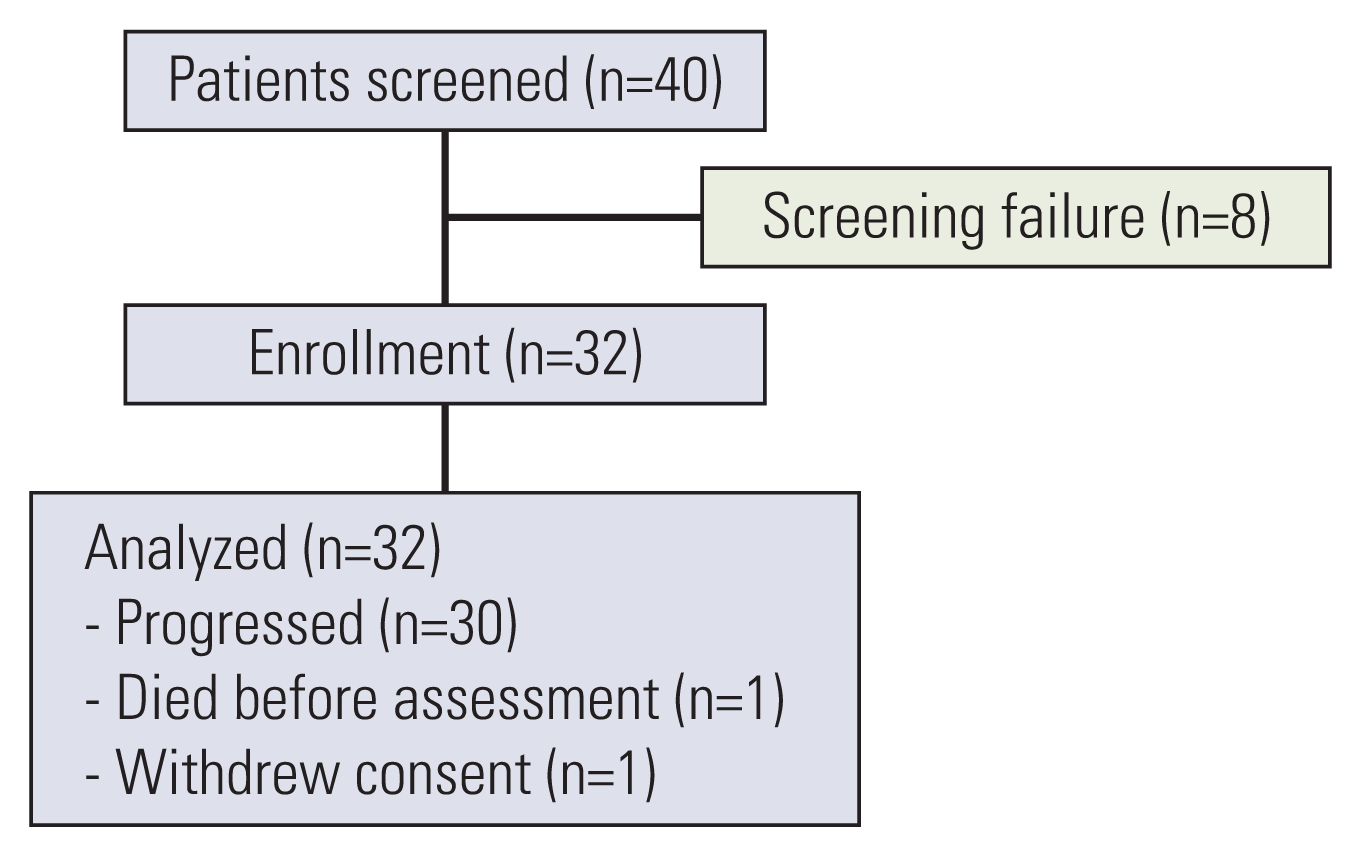
Between July 2016 and November 2019, we screened 40 patients with advanced SqCC and enrolled 32 patients to receive pemetrexed as salvage chemotherapy (Fig. 1). After enrollment, one patient withdrew consent following treatment initiation, and one patient died after receiving the first cycle of pemetrexed but before the tumor assessment.
The clinical characteristics of the 32 patients enrolled in this study are listed in Table 1. Overall, the median patient age was 67 years (range, 56 to 84 years), and 96.9% were male. The ECOG performance status at enrollment was 0 in 15 patients (46.9%), 1 in nine patients (28.1%), and 2 in eight patients (25.0%). All participants were smokers with a median of 42 pack-years (range, 16 to 111). Seven patients (21.9%) had received three or more lines of chemotherapy prior to enrollment. The level of TS expression was negative in 22 patients (68.8%) and weakly positive in 10 patients (31.2%). Tissue specimens were collected via biopsy in 17 patients (53.1%), aspiration in 12 patients (37.5%), and surgical resection in three patients (9.4%).
Tumor response was evaluable in 30 patients. No objective response was observed. Twenty patients (66.7%) had progressive disease and 10 patients had stable disease as their best response. Thus, the disease control rate was 33.3%.
As of the data cutoff date of November 1, 2019, 10 patients were known to be alive, and the median follow-up duration was 19.6 months (95% confidence interval [CI], 9.4 to 29.7). The 6-week and 12-week PFS rates were 35.0% (95% CI, 21.5 to 56.8) and 24.5% (95% CI, 13.0 to 46.1), respectively. The median PFS was 1.3 months (95% CI, 1.3 to 2.7), and the median OS was 11.8 months (95% CI, 8.1 to not applicable).
S1 Table shows adverse event data. Most adverse events were grade 1 or 2. The most common toxicity was rash, with an incidence of 21.9%, followed by constipation, aspartate aminotransferase elevation, and electrolyte imbalance, each of which occurred in four patients (12.5%). Only two grade 3 adverse events occurred, one of which was skin rash and the other serum creatinine elevation. No grade 4 toxicity was noted in this study.
To the best of our knowledge, the present study is the first to evaluate the antitumor activity of pemetrexed in lung SqCC patients with low TS expression. Among the 32 patients included in the study, 25.0% had an ECOG performance status of 2, and 21.9% had received three or more lines of chemotherapy. Patients with the TS-negative SqCC represented 28.8% of the total number enrolled. Pemetrexed exhibited a disease control rate of 30.0%, a 12-week PFS rate of 24.5%, and a median PFS of 1.3 months. The observed adverse effects of pemetrexed in this study were consistent with the profile reported in previous studies [9,11]. The present study demonstrated that pemetrexed was well tolerated but ineffective in improving the clinical outcomes of heavily-treated patients with TS-low SqCC of the lung.
Two prospective trials in patients with nonsquamous NSCLC have shown differential responses to pemetrexed based on TS expression level, which supported the use of TS expression as a predictive biomarker for pemetrexed efficacy [17,23]. Considering the previous findings, how can pemetrexed’s lack of efficacy against TS-low SqCC in our trial be interpreted?
First, pemetrexed resistance in lung SqCC is likely too complex to be explained by TS overexpression alone. Hou et al. predicted pemetrexed response using the expression signatures of 25 genes encoding target enzymes or other molecules related to the drug, including the gene encoding TS [24]. The authors showed that TS expression alone failed to correlate with pemetrexed sensitivity in NSCLC cell lines. A recent review also suggested that pemetrexed resistance in NSCLC may involve numerous potential mechanisms associated with altered pharmacodynamics of the drug or the activation of pathways that might bypass its action [25]. For instance, pemetrexed resistance can result from impaired cellular uptake via certain folate receptors, defective intracellular polyglutamylation, enhanced efflux of the drug, and elevated levels of its target enzymes. These findings indicate the presence of additional causative factors at play for pemetrexed sensitivity.
Second, the meaning of a molecular marker may differ depending on its histological context. The clinical studies that showed TS expression to be a predictive marker of pemetrexed efficacy were conducted in patients with nonsquamous NSCLC [17,23]. TS expression may predict pemetrexed efficacy only in patients with nonsquamous NSCLC, whereas it may not be predictive of pemetrexed response in patients with SqCC. A representative example showing the critical role of histology in predicting drug sensitivity is the BRAFV600E mutation, which is a well-known predictive marker for BRAF-targeted inhibitors. Whereas vemurafenib, a BRAF inhibitor, demonstrated an objective response rate (ORR) of 48% in patients with BRAFV600E-mutated melanoma [26], it showed minimal response in patients with colorectal cancer harboring the BRAFV600E mutations (ORR < 5%) [27]. The mechanism underlying the tissue-specific primary drug resistance involves feedback activation of epidermal growth factor receptor in patients with colorectal cancer, which is not observed in those with melanoma [28]. Likewise, molecular mechanisms specific to lung SqCC may underlie its primary resistance to pemetrexed.
The lack of objective response observed in our study can be partly explained by the relative insensitivity of the study participants, given the previously reported ORR of 2.8% for pemetrexed in the second-line treatment of patients with SqCC [9,12]. Indeed, substantial proportions of our study population had a poor performance status and had received multiple lines of chemotherapy. Of the patients enrolled in our study, 25% had performance status of 2 and 50% had previously undergone more than one line of chemotherapy. Poor performance status is an independent prognostic factor for short survival in patients with NSCLC [29], and heavily-pretreated patients may exhibit more aggressive tumor biology than their counterparts.
Of note, one may question whether IHC is an optimal method for evaluating TS expression. Although quantitative reverse transcription polymerase chain reaction (qRT-PCR) is highly sensitive and has been used for many preclinical studies on TS, it is hardly used in routine practice settings due to its high cost, relative technical complexity, and requirement for fresh tissue. In this context, Ceppi et al. [18] reported a strong correlation between mRNA and protein expression levels, assessed by qRT-PCR and IHC, respectively. Furthermore, low TS expression by IHC was shown to be associated with favorable clinical outcome in the two prospective trials with nonsquamous NSCLC patients [17,23].
Our study has several limitations. First, the limited number of patients included in the study may have prevented us from detecting subtle differences. Second, the lack of comparator arm could have made our hypothesis testing less robust. Third, the TS expression level assessed from biopsy specimens might not be representative of the TS level at a given time or overall TS level of the tumor in an individual patient. Expression levels of certain proteins may vary throughout the tumor as intratumoral heterogeneity develops temporally and spatially [30]. Finally, although we attempted to adjust for potential confounders, residual confounding by unrecorded variables cannot be ruled out completely. Despite these limitations, the present study is the first prospective study of pemetrexed in biomarker-selected patients with squamous NSCLC.
In conclusion, pemetrexed was not active against TS-low SqCC of the lung in a salvage setting, although its toxicity was generally manageable. Future studies are warranted to identify active and safe new agents for treating patients with SqCC of the lung.
Electronic Supplementary Material
Supplementary materials are available at Cancer Research and Treatment website (https://www.e-crt.org).
Notes
Ethical Statement
All patients provided written informed consent. The study protocol was reviewed and approved by the Institutional Review Board of the National Cancer Center, Korea (NCC2016-0108).
ACKNOWLEDGMENTS
This study was supported by National Cancer Center Research grant (1710230).
We thank the patients and their families for participating in this study. We also thank the clinical research nurses of Center for Lung Cancer in the National Cancer Center Korea for administering drugs and acquiring samples.
References
1. Travis WD. Lung cancer pathology: current concepts. Clin Chest Med. 2020; 41:67–85.
2. Sogaard M, Thomsen RW, Bossen KS, Sorensen HT, Norgaard M. The impact of comorbidity on cancer survival: a review. Clin Epidemiol. 2013; 5:3–29.

3. Putila J, Guo NL. Combining COPD with clinical, pathological and demographic information refines prognosis and treatment response prediction of non-small cell lung cancer. PLoS One. 2014; 9:e100994.

4. Heist RS, Sequist LV, Engelman JA. Genetic changes in squamous cell lung cancer: a review. J Thorac Oncol. 2012; 7:924–33.

5. Cancer Genome Atlas Research Network. Comprehensive genomic characterization of squamous cell lung cancers. Nature. 2012; 489:519–25.
6. Horn L, Spigel DR, Vokes EE, Holgado E, Ready N, Steins M, et al. Nivolumab versus docetaxel in previously treated patients with advanced non-small-cell lung cancer: two-year outcomes from two randomized, open-label, phase III trials (CheckMate 017 and CheckMate 057). J Clin Oncol. 2017; 35:3924–33.

7. Paz-Ares L, Luft A, Vicente D, Tafreshi A, Gumus M, Mazieres J, et al. Pembrolizumab plus chemotherapy for squamous non-small-cell lung cancer. Network Engl J Med. 2018; 379:2040–51.

8. Reck M, Rodriguez-Abreu D, Robinson AG, Hui R, Csoszi T, Fulop A, et al. Updated analysis of KEYNOTE-024: pembrolizumab versus platinum-based chemotherapy for advanced non-small-cell lung cancer with PD-L1 tumor proportion score of 50% or greater. J Clin Oncol. 2019; 37:537–46.

9. Hanna N, Shepherd FA, Fossella FV, Pereira JR, De Marinis F, von Pawel J, et al. Randomized phase III trial of pemetrexed versus docetaxel in patients with non-small-cell lung cancer previously treated with chemotherapy. J Clin Oncol. 2004; 22:1589–97.

10. Scagliotti GV, Parikh P, von Pawel J, Biesma B, Vansteenkiste J, Manegold C, et al. Phase III study comparing cisplatin plus gemcitabine with cisplatin plus pemetrexed in chemotherapy-naive patients with advanced-stage non-small-cell lung cancer. J Clin Oncol. 2008; 26:3543–51.

11. Ciuleanu T, Brodowicz T, Zielinski C, Kim JH, Krzakowski M, Laack E, et al. Maintenance pemetrexed plus best supportive care versus placebo plus best supportive care for non-small-cell lung cancer: a randomised, double-blind, phase 3 study. Lancet. 2009; 374:1432–40.

12. Scagliotti G, Hanna N, Fossella F, Sugarman K, Blatter J, Peterson P, et al. The differential efficacy of pemetrexed according to NSCLC histology: a review of two Phase III studies. Oncologist. 2009; 14:253–63.

13. Scagliotti G, Brodowicz T, Shepherd FA, Zielinski C, Vansteenkiste J, Manegold C, et al. Treatment-by-histology interaction analyses in three phase III trials show superiority of pemetrexed in nonsquamous non-small cell lung cancer. J Thorac Oncol. 2011; 6:64–70.

14. Chattopadhyay S, Moran RG, Goldman ID. Pemetrexed: biochemical and cellular pharmacology, mechanisms, and clinical applications. Mol Cancer Ther. 2007; 6:404–17.

15. Ozasa H, Oguri T, Uemura T, Miyazaki M, Maeno K, Sato S, et al. Significance of thymidylate synthase for resistance to pemetrexed in lung cancer. Cancer Sci. 2010; 101:161–6.

16. Liu Y, Yin TJ, Zhou R, Zhou S, Fan L, Zhang RG. Expression of thymidylate synthase predicts clinical outcomes of pemetrexed-containing chemotherapy for non-small-cell lung cancer: a systemic review and meta-analysis. Cancer Chemother Pharmacol. 2013; 72:1125–32.

17. Sun JM, Ahn JS, Jung SH, Sun J, Ha SY, Han J, et al. Pemetrexed plus cisplatin versus gemcitabine plus cisplatin according to thymidylate synthase expression in nonsquamous non-small-cell lung cancer: a biomarker-stratified randomized phase II trial. J Clin Oncol. 2015; 33:2450–6.

18. Ceppi P, Volante M, Saviozzi S, Rapa I, Novello S, Cambieri A, et al. Squamous cell carcinoma of the lung compared with other histotypes shows higher messenger RNA and protein levels for thymidylate synthase. Cancer. 2006; 107:1589–96.

19. Tanaka F, Wada H, Fukui Y, Fukushima M. Thymidylate synthase (TS) gene expression in primary lung cancer patients: a large-scale study in Japanese population. Ann Oncol. 2011; 22:1791–7.

20. Eisenhauer EA, Therasse P, Bogaerts J, Schwartz LH, Sargent D, Ford R, et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer. 2009; 45:228–47.

21. Niyikiza C, Baker SD, Seitz DE, Walling JM, Nelson K, Rusthoven JJ, et al. Homocysteine and methylmalonic acid: markers to predict and avoid toxicity from pemetrexed therapy. Mol Cancer Ther. 2002; 1:545–52.
22. Mandrekar SJ, Qi Y, Hillman SL, Allen Ziegler KL, Reuter NF, Rowland KM Jr, et al. Endpoints in phase II trials for advanced non-small cell lung cancer. J Thorac Oncol. 2010; 5:3–9.

23. Nicolson MC, Fennell DA, Ferry D, O’Byrne K, Shah R, Potter V, et al. Thymidylate synthase expression and outcome of patients receiving pemetrexed for advanced nonsquamous non-small-cell lung cancer in a prospective blinded assessment phase II clinical trial. J Thorac Oncol. 2013; 8:930–9.

24. Hou J, Lambers M, den Hamer B, den Bakker MA, Hoogsteden HC, Grosveld F, et al. Expression profiling-based subtyping identifies novel non-small cell lung cancer subgroups and implicates putative resistance to pemetrexed therapy. J Thorac Oncol. 2012; 7:105–14.

25. Liang J, Lu T, Chen Z, Zhan C, Wang Q. Mechanisms of resistance to pemetrexed in non-small cell lung cancer. Transl Lung Cancer Res. 2019; 8:1107–18.

26. Chapman PB, Hauschild A, Robert C, Haanen JB, Ascierto P, Larkin J, et al. Improved survival with vemurafenib in melanoma with BRAF V600E mutation. N Engl J Med. 2011; 364:2507–16.

27. Kopetz S, Desai J, Chan E, Hecht JR, O’Dwyer PJ, Maru D, et al. Phase II pilot study of vemurafenib in patients with metastatic BRAF-mutated colorectal cancer. J Clin Oncol. 2015; 33:4032–8.

28. Prahallad A, Sun C, Huang S, Di Nicolantonio F, Salazar R, Zecchin D, et al. Unresponsiveness of colon cancer to BRAF(V600E) inhibition through feedback activation of EGFR. Nature. 2012; 483:100–3.

Table 1
Patient characteristics




 PDF
PDF Citation
Citation Print
Print


 XML Download
XML Download