Abstract
Background
Approximately one-fourth of admissions to stroke centers are diagnosed with non-stroke conditions or stroke mimics. Differentiating between these diagnoses and acute ischemic stroke is an important and time-sensitive task. The decision of whether or not to administer thrombolytic therapy is also a critical component, and its safety has been studied numerous times.
Case Report
This case presents a patient with pneumococcal meningitis initially diagnosed as an acute ischemic stroke treated with thrombolytic therapy before further imaging.
Conclusion
Many stroke mimics such as migraines, infections, and seizures exist. Time is of the essence for the treatment of an acute ischemic stroke. The safety profile of tissue plasminogen activator has been studied numerous times in stroke mimics and shown to be relatively safe indicating if the patient has no contraindications for stroke intervention, treatment of stroke should not be extensively delayed to rule out stroke confounders.
Stroke continues to be one of the leading causes of morbidity and mortality in the United States. Guidelines from the American Stroke Association emphasize the importance of quickly recognizing and initiating treatment. The National Institutes of Health Stroke Scale (NIHSS) is one of the measures used to identify characteristics of strokes such as deficits in language, motor, and sensory functions. Intravenous (IV) tissue plasminogen activator (tPA) is a treatment option utilized in acute ischemic strokes (AISs) with a window of time of 3 hours that can be extended to 4.5 hours from the initiation of symptoms [1]. Clinical outcome measures have identified a door-to-needle time of 60 minutes or less defined as the time from when the patient enters the hospital to when treatment is administered. This necessitates a rapid identification of AISs [2]. While IV tPA remains the standard of care for AIS, new studies have demonstrated the benefits of endovascular intervention along with intraarterial tPA administration and mechanical thrombectomy [3]. Several conditions confound and mimic stroke symptoms including seizures, syncope, conversion disorder, metabolic disorders, infections, and migraines [4]. These conditions can cause misidentification and the initiation of ineffective treatments. This case is an example of such a confounder in a patient who presented with aphasia, dysarthria, and confusion and was treated as an AIS but further imaging indicated otomastoiditis, pneumocephalus, and pneumococcal meningitis. This case highlights the safety of tPA in a stroke mimic case with a patient who required surgery for an intracranial process diagnosed after tPA administration and did not suffer any complications due to thrombolytic therapy administration. Written consent was obtained from the patient before the publication of this case report.
A 65-year-old female with a past medical history of end-stage renal disease (ESRD) on daily peritoneal dialysis, hypertension, rectal cancer, chronic variable immunodeficiency with a history of receiving subcutaneous immunoglobulin infusions, history of rheumatic fever, history of non-ST elevated myocardial infarction, asthma, hypothyroidism, gastroesophageal reflux disease, and recent 1-month history of otitis media treated with an 8-day course of cephalexin presented to an outside hospital with expressive aphasia, confusion, dysarthria, and decreased level of consciousness. She presented to the hospital approximately 3 hours after the onset of symptoms. Initial NIHSS was 7. The patient received a computed tomography head performed at the outside hospital, which was negative for intracranial abnormality, per report. The patient’s symptoms began waning while being managed in the emergency room; however, the decision to administer a 15 mg IV tPA bolus followed by an infusion was made. This was administered approximately 4 hours and 17 minutes after the onset of symptoms. Before transfer, her NIHSS was 3.
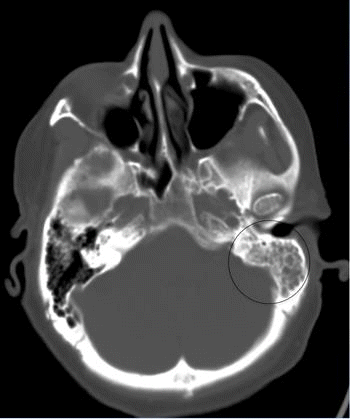
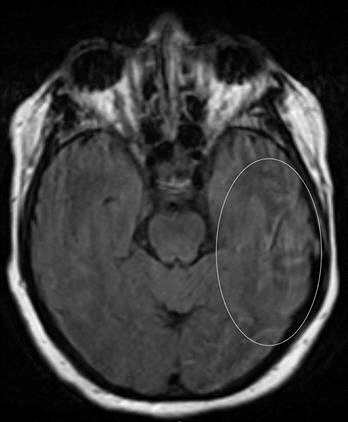
Upon arrival to our facility, NIHSS was 2 for expression aphasia and confusion with Glasgow Coma Scale of 14 with no noted cranial nerve abnormalities. An emergent computed tomography angiography of the head and neck was performed which indicated no hemorrhage, mass, infarct, or vascular occlusion. However, the scan did note complete opacification of left mastoid air cells and left temporal pneumocephalus suspicious for acute otomastoiditis (Fig. 1). Following the report of this scan, a magnetic resonance imaging of the head was ordered which noted nonsuppression of fluid-attenuated inversion recovery around the left temporal and occipital lobes suspicious for meningitis. (Fig. 2) Consults to infectious disease and otolaryngology were placed immediately. Upon further exam of bilateral ears, the patient was noted to have left ear opacification with purulent effusion with Weber tuning fork test lateralizing to the left ear indicative of left bone conduction greater than air conduction.
On the day of admission, the patient was started on renally dosed vancomycin and 1 g IV cefepime every 24 hours. There was no leukocytosis nor elevated temperatures throughout the hospital admission. A lumbar puncture was unable to be obtained due to the timing of IV tPA administration. In addition, 20 g IV immunoglobulin was administered since the patient had not received subcutaneous immunoglobulin for 10 months before presentation due to ESRD. Tympanomastoidectomy and myringotomy with tube placement and Penrose drain placement were performed on hospital day 2. Granulation tissue was noted through the middle ear as well as mastoid and tegmen dehiscence. Cultures collected indicated numerous (20–50/oil immersion field) white blood cells, moderate (5–10/oil immersion field) Gram-positive cocci in pairs, which were later identified as Streptococcus pneumoniae. Antibiotics were adjusted to renally dosed vancomycin and 2 g ceftriaxone every 12 hours on the day of surgery. The patient’s neurologic status returned to baseline after surgery, and no neurological deficits were present on discharge. The Penrose drain was removed on postoperative day 2 and ciprofloxacin otic drops were initiated in the left ear for 1 week. The patient underwent a procedure for Hickman catheter placement in order to receive outpatient antibiotic treatment and was discharged from the hospital on a 2-week course of ceftriaxone 2 g IV every 12 hours and vancomycin 1.25 g every 72 hours.
This case involves pneumococcal meningitis presenting with symptoms resembling an AIS. The leading cause of bacterial meningitis in adults is S. pneumoniae [5]. In a review by Durand et al. [5], only two-thirds of patients exhibited the triad of fever, change in mental status, and nuchal rigidity characteristic of bacterial meningitis. Headache, neck stiffness, altered mental status, temperature >38,oC were the most common symptoms of presentation; however, seizures, tachycardia, hypotension, and focal neurological abnormalities were also reported in a large prospective cohort study by Weisfelt et al. [6]. Aphasia, one of the presenting symptoms in our case, was present in 34% patients in this study. Otitis and sinusitis were the most common predisposing condition manifesting in 43% of patients [6]. Uncommon symptoms have been shown to manifest in the elderly and immunocompromised. Otitis intracranial complications have been shown in adult patients to mimic stroke symptoms with symptoms such as hemiparesis and decreased level of consciousness [7].
Pneumocephalus is defined as air within the intracranial compartments. Otogenic pneumocephalus, which was described in our case, is a rare and severe manifestation of pneumococcal meningitis, which has been described previously in several case reports [8,9]. In a study by Cuinat et al. [10], the meningeal disease was found to be the diagnosis in 1.7% of patients presenting with stroke symptoms while 25.3% of the patients overall were found to have a non-stroke diagnosis.
Many confounders and stroke mimics exist clouding a stroke diagnosis. Dawson et al. [4] found that 24.2% of patients admitted to a stroke center were diagnosed as stroke mimics. Among these, headaches, seizures, sepsis, and syncope were the most common symptoms [4,11]. Other confounders include metabolic disorders such as hypoglycemia, neuropathy, drug intoxication, conversion disorder, dementia, or brain masses [11]. The concern over these confounders is two-fold involving diversion of stroke care resources and facilities to non-stroke conditions and the inappropriate treatment of non-stroke conditions with thrombolytics [12]. In addition, there has been concern that with the number of stroke mimics, actual stroke diagnoses will be missed in favor of a non-stroke diagnosis. Stroke mimics along with stroke chameleons, defined as strokes with unusual presenting symptoms, could be harmful because of the delay in or lack of administration of stroke medications and interventions [12]. Two cases describing both the medical and legal consequences of strokes misdiagnosed as mimics leading to severe medical and legal outcomes are described by Moore et al. [13].
Due to the concern of treating patients who may end up being diagnosed as a stroke mimic, several studies involving the safety of tPA have been conducted. In a study described by Chernyshev et al. [14], 14% of patients who received tPA at their facility ended up being diagnosed with a stroke mimic diagnosis. However, none of these patients were found to have a symptomatic intracranial hemorrhage (sICH) as a complication of the thrombolytic therapy while patients diagnosed with an AIS in this study exhibited a 6% risk of an sICH as a complication of tPA therapy [14]. In a large multicenter cohort study, Ali-Ahmed et al. [15] found that 3.5% of patients who received tPA were stroke mimics. The rate of sICH was found to be 0.4% in the stroke mimic group and 3.5% in the AIS group [15]. The patient in our study initially received tPA before further imagining revealing otomastoiditis, pneumocephalus, and pneumococcal meningitis and did not have an sICH as a complication. The patient’s symptoms did improve after IV tPA from NIHSS 7 to NIHSS 2 indicating that this patient may have experienced a thrombotic event secondary to the inflammation from mastoiditis/meningitis which was amenable to IV tPA. These studies demonstrate that although risks of administering thrombolytics are not nonexistent, therapy should not be delayed for an extended time in order to confirm stroke diagnosis and rule out stroke mimics.
Stroke mimics include diagnoses such as migraines, infections, and seizures which present with stroke-like symptoms. Our case highlights such a mimic in a patient who presented with aphasia, dysarthria, and confusion and initially was diagnosed as an AIS receiving tPA therapy. While obtaining a history, physical, and imaging are critical to the diagnosis of a patient presenting with stroke-like symptoms, time is of the essence for treatment. Several studies have indicated the relatively safe profile of tPA in stroke mimics indicating if the patient has no contraindications for stroke intervention, treatment of stroke should not be extensively delayed to rule out stroke confounders [14,15].
Notes
Ethics statement
Approval for this study was waived in accordance with The University of Kansas policies because this study is a case report of a single patient and did not include protected health information, data analysis, or testing of a hypothesis, and was de-identified. Written consent was obtained from the patient before the publication of this case report.
REFERENCES
1. Powers WJ, Rabinstein AA, Ackerson T, Adeoye OM, Bambakidis NC, Becker K, et al. 2018 Guidelines for the Early Management of Patients With Acute Ischemic Stroke: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2018; 49:e46–110.

2. Fonarow GC, Zhao X, Smith EE, Saver JL, Reeves MJ, Bhatt DL, et al. Door-to-needle times for tissue plasminogen activator administration and clinical outcomes in acute ischemic stroke before and after a quality improvement initiative. JAMA. 2014; 311:1632–40.

3. O'Carroll CB, Rubin MN, Chong BW. What is the role for intra-arterial therapy in acute stroke intervention? Neurohospitalist. 2015; 5:122–32.
4. Dawson A, Cloud GC, Pereira AC, Moynihan BJ. Stroke mimic diagnoses presenting to a hyperacute stroke unit. Clin Med (Lond). 2016; 16:423–6.

5. Durand ML, Calderwood SB, Weber DJ, Miller SI, Southwick FS, Caviness VS Jr, et al. Acute bacterial meningitis in adults: a review of 493 episodes. N Engl J Med. 1993; 328:21–8.
6. Weisfelt M, van de Beek D, Spanjaard L, Reitsma JB, de Gans J. Clinical features, complications, and outcome in adults with pneumococcal meningitis: a prospective case series. Lancet Neurol. 2006; 5:123–9.

7. Van der Poel NA, van Spronsen E, Dietz de Loos DA, Ebbens FA. Early signs and symptoms of intracranial complications of otitis media in pediatric and adult patients: a different presentation? Int J Pediatr Otorhinolaryngol. 2017; 102:56–60.

8. Pantangi P, Cherian SV. Pneumocephalus; a rare presentation of streptococcal meningitis. Intern Med. 2011; 50:2249–50.

9. Damergis JA, Chee K, Amitai A. Otogenic pneumococcal meningitis with pneumocephalus. J Emerg Med. 2010; 39:e109–12.

10. Cuinat L, Nasr N, Kamsu JM, Tanchoux F, Bonneville F, Larrue V. Meningeal disease masquerading as transient ischemic attack. J Stroke Cerebrovasc Dis. 2014; 23:1738–43.

11. Fernandes PM, Whiteley WN, Hart SR, Al-Shahi Salman R. Strokes: mimics and chameleons. Pract Neurol. 2013; 13:21–8.

13. Moore MJ, Stuart J, Humphreys A, Pfaff JA. To tPA or not to tPA: two medical-legal misadventures of diagnosing a cerebrovascular accident as a stroke mimic. Clin Pract Cases Emerg Med. 2019; 3:194–8.





 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download