Abstract
Hypertension is a major, yet manageable, risk factor for stroke, and the benefits of well-controlled blood pressure are well established. However, the strategy for managing blood pressure can differ based on the pathomechanism (subtype), stage, and treatment of stroke patients. In the present review, we focused on the management of blood pressure during the acute stage of intracerebral hemorrhage, subarachnoid hemorrhage, and cerebral infarction. In patients with cerebral infarction, the target blood pressure was discussed both before and after thrombolysis or other endovascular treatment, which may be an important issue. When and how to start antihypertensive medications during the acute ischemic stroke period were also discussed. In regards to the secondary prevention of ischemic stroke, the target blood pressure may differ based on the mechanism of ischemic stroke. We have reviewed previous studies and guidelines to summarize blood pressure management in various situations involving stroke patients.
Stroke is a disease with a heterogeneous pathomechanism, and is primarily considered to be hemorrhagic or ischemic. Hemorrhagic stroke is the result of ruptured blood vessels in the brain, resulting in intracerebral hemorrhage (ICH). This includes ruptured perforator vessels in the deep structures of the brain, including the basal ganglia, thalamus, pons, and cerebellum [1]. In cases of lobar hemorrhage, two potential causes are underlying cerebral amyloid antipathy or cancer metastases. The rupture of an intracranial aneurysm may lead to subarachnoid hemorrhage (SAH), which has a high mortality rate during the acute phase [2]. The mechanism of ischemic stroke is more heterogeneous, compared to hemorrhagic stroke. Embolisms caused by large artery atherosclerosis in the proximal vessels can cause infarction. The heart chambers may be one such source of embolism (cardioembolism). Another example of this is lacunar infarction, which results from the occlusion of small perforators due to lipohyalinosis, which subsequently causes small infarctions [3].
Hypertension is one of the most important risk factors for stroke, and is the risk factor with the highest population-attributable risk [4]. High blood pressure (BP) may lead to endothelial dysfunction, resulting in the development of atherosclerosis [5]. Hypertension also affects the heart directly and increases the risk of cardioembolic stroke [6]. Additionally, small perforators originating from the intracranial artery are affected by hypertension. In contrast to capillary vessels, the perforator branches are perpendicular to their source vessels, and are characterized by a sudden decrease in diameter, which makes them vulnerable to increased BP. High BP may cause these small perforators to rupture, leading to ICH, or may cause occlusion, resulting in lacunar infarction. Therefore, lowering BP is highly effective for the prevention of secondary stroke and acute complications of ICH [7]. However, the target BP may differ slightly per patient, based on the stroke mechanism.
Furthermore, the goal of BP control may differ based on the stage (acute vs. chronic) and treatment (thrombolysis or thrombectomy) of stroke. In this comprehensive review, we aimed to discuss BP control in a variety of situations involving stroke.
Hemorrhagic strokes are categorized as either ICH or SAH, with ICH accounting for 10% to 20% of all strokes, and SAH accounting for approximately 5% [8]. Hemorrhagic stroke occurs less frequently than ischemic stroke, but the morbidity and mortality are still considerable. Since patients often deteriorate rapidly within a few hours after onset, appropriate management in the acute stage is important. The primary medical treatment for acute spontaneous ICH is BP management, in which the goals are to reduce hematoma expansion and perihematomal edema, improving the functional outcome and reducing mortality. In patients with SAH, BP should be controlled to balance the risk of rebleeding while maintaining cerebral perfusion pressure (CPP).
Increased BP is very common in ICH, which may stem from premorbid hypertension or secondary increase due to increased intracranial pressure (ICP), stress, or pain [9]. Elevated BP during the acute phase of ICH has been found to be associated with hematoma expansion, perihematomal edema, rebleeding, neurological deterioration, and death [10,11]. However, there are concerns about decreasing BP too far, which may cause cerebral ischemia around the hematoma. To evaluate this concern, a randomized clinical trial (RCT) was conducted to measure cerebral blood flow (CBF) following a decrease in BP [12]. Participants included patients within 24 hours of onset of acute ICH, with a systolic BP (systolic blood pressure [SBP]) >150 mmHg. The patients were divided into two groups, with targeted SBPs of <150 mmHg and <180 mmHg. Perfusion computed tomography (CT) imaging was used to compare the CBF around the hematomas. It was found that the degree of BP reduction did not affect the CBF around the hematoma, and thus, it the reduction was safe.
Two large clinical trials have evaluated the efficacy of the intensive lowering of BP in patients with acute ICH [13,14]. First, the Intensive Blood Pressure Reduction in Acute Cerebral Hemorrhage-2 (INTERACT-2) trial evaluated 2,839 patients with SBPs between 150 and 220 mmHg within 6 hours of ICH onset, who were randomized into groups with target SBPs of <140 or <180 mmHg [13]. The primary outcome was death or major disability, defined as a modified Rankin scale (mRS) score ≥3, which did not differ significantly between the two groups. This was evidenced by an odds ratio (OR) of 0.87 in the intensive treatment group, a 95% confidence interval (CI) of 0.75–1.01, and a P=0.06. However, the intensive treatment group had better functional recovery and an improved physical and mental health-related quality of life compared to the standard treatment group. Additionally, the drastic reduction in BP did not cause serious adverse events. In concurrence with these results, the American Heart Association (AHA) and American Stroke Association (ASA) have provided evidence-based consensus guidelines which state that for patients with an SBP between 150 and 220 mmHg and without contraindications, the acute lowering of SBP to 140 mmHg is considered safe and may improve functional outcomes in patients with ICH [15].
Another trial, the Antihypertensive Treatment of Acute Cerebral Hemorrhage-2 (ATACH-2) trial, was published after the publication of these AHA/ASA guidelines [14]. This randomized trial evaluated patients with acute ICH and hypertension, assigning them to groups with targeted SBPs of 110–139 and 140–179 mmHg, however, antihypertensive treatment had to be initiated within 4.5 hours of the onset of ICH. The trial was terminated early due to futility, after an interim analysis demonstrated that the rates of death or severe disability (mRS ≥4) at three months were similar in both groups (relative risk, 1.04 in intensive treatment group; 95% CI, 0.85–1.27; P=0.72). Unlike the INTERACT-2 trial, ATACH-2 showed no difference in the ordinal distribution of mRS scores, but did show a higher rate of adverse renal events within 1 week in the intensive treatment group than was found in the standard treatment group (9.0% vs. 4.0%, P=0.002). This discrepancy was due to the excessive lowering of BP on the first day. The results of that study suggested that rapid and aggressive BP reduction could result in end-organ damage [16].
The difference between the results of these two trials can be attributed to the difference in both the degree and rate of BP reduction. Although the target SBPs were the same in both trials, the actual BP reduction was faster and more pronounced in ATACH-2 (mean minimum SBP during the first 2 hours, 128.9 mmHg in ATACH-2 vs. 141.1 mmHg in INTERACT-2). This means that the SBP for the standard treatment group in the ATACH-2 trial was similar to those for the intensive treatment group in INTERACT-2 (Table 1). Additionally, SBP <130 mmHg were also associated with worse prognoses in the post-hoc analysis of INTERACT-2 [17]. Therefore, it was suggested that a SBP of approximately 140 mmHg, rather than rapidly lowering the SBP to below 130 mmHg, may be the optimal SBP target.
A recent retrospective study suggested a potentially lower SBP limit for BP management in acute ICH [16]. When comparing the target SBP <160 mmHg group and the SBP <140 mmHg group, acute cerebral ischemia and acute neurological deterioration were more common in the SBP <140 mmHg group. More specifically, cerebral ischemia increased when the minimum SBP <120 mmHg was observed for more than 72 hours. In the case of a minimum SBP >130 mmHg, no patients showed additional cerebral ischemia. Thus, that study suggested the possibility that an SBP of 130 to 140 mmHg would be an appropriate target SBP.
Few studies have been done regarding the safety and effectiveness of BP-lowering therapy in patients with extremely elevated BP (sustained SBP >220 mmHg) as related to symptom presentation. The AHA/ASA guidelines recommend that the aggressive reduction of BP with continuous intravenous infusion and frequent BP monitoring may be reasonable for such patients. Additionally, the optimal time at which to start lowering BP to prevent recurrent stroke is not well known; however, starting BP management with a target BP of <130/80 mmHg is recommended when the patient is medically and neurologically stable [15].
Based on the currently available information, the optimal management of hypertension in acute ICH remains unclear. Large RCTs did not provide consistent evidence that a specific target BP is beneficial, and provided information that rapid and aggressive BP reduction can be harmful. Ultimately, individualization of the BP target, taking into account the risk and benefit in each patient, may be needed.
In addition to absolute SBP levels, BP variability may predict poor clinical outcomes in ICH, although the exact mechanism by which BP variability affects poor outcomes in patients with ICH is not fully understood [18]. Recurrent excessive BP fluctuations may increase oncotic and hydrostatic pressure gradients in the perihematomal region, and subsequently enhance perihematomal edema. Furthermore, these fluctuations may be associated with autonomic dysfunction that promotes proinflammatory cytokine production, hyperglycemia, disruption of the blood-brain barrier, and vasogenic edema, all of which may contribute to worse outcomes in patients with ICH [19].
Rebleeding in patients with SAH leads to an extremely poor prognosis. Although proper BP control is necessary to prevent rebleeding, the magnitude of BP control necessary to reduce the risk of rebleeding has not been established. For most patients with acute SAH, the AHA/ASA recommends maintaining a SBP <160 mmHg to balance the risks of ischemia and rebleeding [20]. The Neurocritical Care Society states that extreme hypertension in SAH should be avoided, and suggests maintaining a mean arterial pressure (MAP) <110 mmHg [21]. When increased ICP is suspected, given the risk of impaired cerebral perfusion, increasing MAP may be the only way to maintain CPP. Therefore, antihypertensive therapy is often withheld unless there is an extreme elevation in BP. European guidelines recommend a MAP above 90 mmHg to maintain proper CPP [22]. Recent studies have reported that BP variability is also associated with rebleeding or other negative outcomes in patients with acute SAH [23,24].
In the case of symptomatic cerebral vasospasm and delayed cerebral ischemia after SAH, induced hypertension is recommended by some guidelines [20,21]. A previous recommendation for triple-H therapy (consisting of hypertension, hemodilution, and hypervolemia) is no longer supported by current guidelines due to the adverse events no known to be associated with hemodilution. Instead, induced hypertension and euvolemia are now recommended [20,21,25]. While an optimal or maximum goal BP has not been established, physicians should consider concomitant cardiac and pulmonary diseases as well as the brain for each patient. Table 2 summarizes BP management in patients with hemorrhagic stroke.
BP management in acute ischemic stroke (AIS) is complex, and is associated with multiple factors. The AHA/ASA guidelines recommend that if AIS patients have a BP >185/110 mmHg and they are eligible for treatment with intravenous alteplase, their BP should be lowered carefully before intravenous alteplase treatment is initiated (class I, level of evidence [LOE] B) [26]. Moreover, current guidelines recommend maintaining BP <180/105 mmHg during and after intravenous alteplase treatment for the first 24 hours after treatment (class I, LOE B) [26]. However, it was not proven by an RCT and was based on the protocol of RCTs. Studies regarding target BP are difficult because it is impossible to blind either the physicians or the patients, and the occurrence of contamination due to lack of true blinding must be considered.
The intensive blood pressure reduction with intravenous thrombolysis therapy for acute ischaemic stroke (ENCHANTED) trial showed that intensive BP control (SBP target 130–140 mmHg) failed to improve the functional outcome (OR, 1.01; 95% CI, 0.87–1.17), but lowered bleeding risk (OR, 0.75; 95% CI, 0.60–0.94) after intravenous thrombolysis treatment compared with guideline-recommended treatment (SBP target <180 mmHg) [27]. However, the ENCHANTED trial did not consider revascularization status, and only 1.9% of the study cohort underwent endovascular treatment. Therefore, the results of that study cannot be applied to patients who have undergone endovascular treatment. Moreover, some observational studies suggest that the risk of hemorrhage after the administration of alteplase is greater in patients with higher BP and BP variability [28]. The exact BP at which the risk of hemorrhage after thrombolysis increases is unknown. It is thus reasonable to target the BPs used in the RCTs involving intravenous thrombolysis, although it may be prudent to consider intensive BP lowering in patients with a high risk of bleeding.
Current AHA/ASA guidelines recommend that for patients who undergo endovascular treatment, BP during and for 24 hours after treatment should be maintained at <180/105 mmHg (class IIa, LOE B). In particular, if successful reperfusion is achieved, guidelines recommend that BP be maintained at <180/105 mmHg (class IIb, LOE B) [26]. This recommendation was based on an endovascular treatment protocol from certain RCTs, although RCT data on optimal BP management are not available yet.
The protocol from the endovascular treatment for small core and anterior circulation proximal occlusion with emphasis on minimizing CT to recanalization times (ESCAPE) trial stated that if reperfusion failed, an SBP ≥150 mmHg may be useful in promoting and maintaining adequate collateral flow, and if successful reperfusion was achieved, normal BP was then targeted [29]. Similarly, in the diffusion-weighted imaging or computerized tomography perfusion assessment with clinical mismatch in the triage of wake up and late presenting strokes undergoing neurointervention (DAWN) trial protocol, a goal SBP <140 mmHg was recommended for patients who achieved successful reperfusion [30]. A recent international multicenter cohort study demonstrated that higher BP within the first 24 hours after successful endovascular treatment was associated with a higher risk of secondary ICH, mortality, and hemicraniectomy [31]. Moreover, when the patients were divided into three groups based on the SBP goal for the first 24 hours after endovascular treatment (<140 mmHg, <160 mmHg, and <180 mmHg), SBP goals of <140 mmHg following successful reperfusion with endovascular treatment appeared to be associated with better clinical outcomes than SBPs <160 and <180 mmHg [32]. Moreover, after reperfusion, lower BP goals have been proposed due to concerns about hemorrhagic complications and reperfusion injury.
However, another recent analysis of individual patient data from three separate RCTs showed that critical MAP thresholds and durations for poor outcome after endovascular treatment were found to be those <70 mmHg for more than 10 minutes and those >90 mmHg for more than 45 minutes [33].
Typically, higher baseline BPs in AIS patients with large vessel occlusions or severe stenosis is associated with better collateral flow. However, a previous study has showed that greater infarct growth was observed in patients without reperfusion, leading to an unfavorable clinical outcome, even in those with a higher baseline BP. Contrarily, a higher baseline BP was associated with decreased infarct growth in patients with successful reperfusion. Therefore, the relationship between baseline BP and outcomes is highly dependent on reperfusion status, and active BP-lowering treatments may be inappropriate in AIS patients prior to reperfusion treatments [34].
In this regard, setting a target BP before and after intravenous thrombolysis treatment and intra-arterial endovascular treatment was difficult. As cerebral autoregulation is impaired in patients with AIS, BP control may be important for improving clinical outcomes. Based on the present knowledge, multiple factors related to clinical outcomes must be considered in order to determine the BP target, including the reperfusion status (successful or not), baseline BP prior to treatment, MAP during treatment, and hemorrhagic transformation after reperfusion treatment (yes or no), and the BP target may be continuously adjusted based on the situation.
An acute hypertensive response can also be observed in patients with AIS. It is usually self-limiting, and the BP spontaneously falls over the week after the onset of stroke [10,35]. However, since the acute hypertensive response to stroke is known to be an independent predictor of poor outcome, it is necessary to maintain optimal BP during the acute stroke period. Many previous trials and studies have investigated the optimal BP level and the effect of early, rapid lowering of elevated BP, however, no ideal BP has been established. A higher BP may be beneficial for the penumbra, which is viable but under-perfused, by increasing collateral flow. On the other hand, a higher BP may increase the risk of hemorrhagic transformation and cerebral edema [36]. In contrast, lowering BP may potentially increase the risk of infarction growth.
BP control recommendations vary depending on the comorbid conditions. According to the AHA/ASA guidelines, early treatment is indicated in patients with severe acute comorbidities such as acute coronary event, acute heart failure, aortic dissection, post-fibrinolysis spontaneous intracranial hemorrhage, hypertensive emergency, or pre-eclampsia/eclampsia (class I, LOE C-EO). An excessive decrease in BP can exacerbate cerebral infarction and should be noted [37], and BP management in these situations should be individualized. Although there is no standard, it is generally considered safe and reasonable to lower BP by 15% from baseline.
For patients who did not receive intravenous alteplase or endovascular treatment and those without comorbid conditions, the recommendations differ based on the BP level. In patients with a BP ≥220/120 mmHg, the benefit of initiating or reinitiating treatment of hypertension within the first 48 to 72 hours is uncertain (class IIb, LOE C-EO). Patients with severe hypertension were excluded from clinical trials, and the effects of rapid BP reduction have not been formally studied. However, it is generally considered reasonable to lower BP by 15% during the first 24 hours after stroke onset.
In patients with a BP <220/120 mmHg, initiating or reinitiating treatment of hypertension within the first 48 to 72 hours after AIS is safe, but not effective in preventing death or dependency (class III: no benefit, LOE A). Except for a few previous studies, the Intravenous Nimodipine West European Stroke Trial (INWEST) [38] and Very Early Nimodipine Use in Stroke (VENUS) [39] trial, which reported that early BP control was associated with worse of outcomes, most RCTs, the Prevention Regimen To Effectively Avoid Second Strokes (PRoFESS) [40], scandinavian candesartan acute stroke trial (SCAST) [41], Controlling Hypertension and Hypotension Immediately Post-Stroke (CHHIPS) [42], Continue or Stop Post-Stroke Antihypertensives Collaborative Study (COSSACS) [43], ENCHANTED [27], and efficacy of nitric oxide, with or without continuing antihypertensive treatment, for management of high blood pressure in acute stroke (ENOS) [44] trials and two meta-analyses [45,46] have consistently shown that initiating or reinitiating antihypertensive therapy within the first 48 to 72 hours after AIS is safe, although this strategy is not associated with improved mortality or functional outcomes. The International Stroke Trial (IST) [35] showed a U-shaped response between BP level and mortality. An SBP level around 150 mmHg was associated with the lowest risk of mortality and poor outcomes of death or dependency.
Persistent hypotension is uncommon in AIS. If a patient does have persistent hypotension, potential caused should be investigated, to look for issues such as aortic dissection, hypovolemia, and decreased cardiac output due to myocardial infarction or arrhythmia. Management of hypotension in patients with AIS has not been well studies. Some observational studies have shown an association between worse outcomes and lower BP, whereas others have not [35,47-49]. The 2019 AHA/ASA guidelines recommend that hypotension and hypovolemia should be corrected to maintain systemic perfusion levels sufficient to support organ function (class I, LOE C-EO).
In patients with a BP >140/90 mmHg who are neurologically stable, starting or restarting antihypertensive therapy during hospitalization is safe (class IIa, LOE B-R) and has been shown to be associated with improved control of BP after discharge in both the COSSACS [43] and china antihypertensive trial in acute ischemic stroke (CATIS) [50] trials. These studies included only patients with a previous diagnosis of hypertension, or enrolled primarily patients with previous hypertension. However, it is also reasonable to apply this recommendation to patients without preexisting hypertension. BP management based on treatment of ischemic stroke is summarized in Fig. 1.
The majority of patients with ischemic stroke have hypertension, and lowering BP may be critical in preventing recurrent stroke. Several RCTs have focused on this issue. The Post-Stroke Antihypertensive Treatment Study (PATS) randomized 5,665 patients with stroke or transient ischemic attack (TIA) into two groups, indapamide 2.5 mg or placebo. After 2 years, indapamide 2.5 mg was found to significantly reduce recurrent stroke, with a hazard ratio (HR)=0.70 and 95% CI, 0.57–0.86 [51]. The Perindopril Protection Against Recurrent Stroke Study (PROGRESS) randomized 6,105 patients with stroke or TIA into two groups, perindopril or placebo. After 4 years, it was found that perindopril reduced recurrent stroke significantly, HR=0.78 and 95% CI, 0.62–0.83 [52]. However, the PRoFESS study randomized 20,322 patients into two groups, telmisartan or placebo, and failed to show a benefit in reducing recurrent stroke or composite vascular events after 2.5 years of follow-up [53]. In a meta-analysis including 10 RCTs with a total of 38,421 patients, lowering BP with medication significantly reduced stroke, OR=0.78 and 95% CI, 0.68–0.90 [54]. Typically, a J-curve was observed from trials on the secondary prevention of coronary artery disease using diastolic BP (DBP). Post-hoc analysis of the PRoFESS study, however, did not reveal a J-curve between BP and recurrent stroke [55]. Furthermore, stroke mortality continuously decreases as SBP decreases under 120 mmHg [56]. However, the effects of and target for BP lowering in ischemic stroke may differ based on the mechanism of stroke.
In patients with symptomatic carotid occlusions, the results of the COSSACS study showed that the effect of lowering BP <130/85 mmHg was a lower risk of ipsilesional ischemic stroke compared to those with BP >130/85 mmHg (HR=0.27; 95% CI, 0.08–0.94) [57]. In another study that included patients with symptomatic carotid stenosis, lowering SBP to 140 mmHg continuously decreased the risk of stroke. BP may be safely lowered to 140 mmHg in those with carotid stenosis. However, it was found that if the stenosis was >70%, the risk of stroke increased as BP decreased. This may be explained by poor collateral flow of the contralateral carotid artery in severe bilateral carotid stenosis [58].
Symptomatic intracranial atherosclerosis is more common than symptomatic extracranial stenosis in patients in Asia. In the post-hoc analysis of Warfarin Aspirin Symptomatic Intracranial Disease (WASID) study, various risk factors were associated with vascular events. Among them, SBP >140 mmHg showed a higher risk of major cardiovascular events (HR=1.79; 95% CI, 1.27–2.52) [59]. In regards to the progression of atherosclerotic burden in intracranial atherosclerosis, the post-hoc analysis of the Trial of Cilostazol in Symptomatic Intracranial Stenosis (TOSS)-2 study showed the lowest rate of progression between 120 and 160 mmHg [60]. The strategy for adequate blood pressure lowering in the patients with intracranial atherosclerosis (STABLE-ICAS) study compared two strategies of BP lowering—intense (target SBP 110–120 mmHg) and standard (target SBP 130–140 mmHg)—on the expansion of white matter hyperintensity lesions. That study was designed as a non-inferiority trial, and failed to show the non-inferiority of intense BP lowering compared to standard BP lowering [61]. There is no evidence based on which to lower BP intensively in patients with intracranial atherosclerosis, and they should be treated with a target SBP between 120 and 140 mmHg.
Small vessel disease is significantly associated with hypertension. The Secondary Prevention of Small Subcortical Strokes (SPS-3) trial was a well-designed RCT focusing on the target BP for lacunar infarction. Using a 2×2 factorial design, the BP arm was an open label trial, comparing the effects of target SBPs of 130–139 and <130 mmHg. The primary endpoint was reduction in stroke, which failed to show a statistical significance between the two groups (HR=0.81; 95% CI, 0.64–1.03). However, the lower target group significantly reduced the risk of ICH (HR=0.37; 95% CI, 0.15–0.95) [7]. Therefore, lowering BP under 130 mmHg may be beneficial in patients with small vessel disease.
Based on these trial results, the ASA/AHA guidelines on the secondary prevention of stroke suggest controlling BP in patients with an established SBP higher than 140 mmHg or DBP higher than 90 mmHg. A reasonable target for lowering BP is <140/90 mmHg. In particular, for patients with small vessel disease or lacunar infarction, a reasonable target BP was suggested as SBP <130 mmHg [62]. Pathophysiology and target BP based on ischemic stroke mechanisms are summarized in Fig. 2.
The management of BP in stroke is complex and challenging due to the variety of stroke subtypes, heterogeneous etiologies, hemodynamic statuses, and comorbidities. Recent data have suggested that lowering BP in acute ICH is probably safe; however, if BP is lowered rapidly in the acute phase, adverse renal events may occur. BP management in AIS remains problematic, and questions such as when to start antihypertensive medications and how significantly to reduce BP have yet to be answered. Large RCTs have not demonstrated the benefit of lowering BP earlier, but the comorbidities, baseline BP, and stroke mechanism should be considered, and the time and target of lowering BP must be individualized based on this information. When reperfusion is achieved through thrombolysis or thrombectomy, lowering BP reduces the risk of hemorrhagic complications. For secondary stroke prevention, BP targets may differ based on the stroke mechanism; intensive lowering of BP may be beneficial in patients with small vessel disease.
Notes
REFERENCES
1. Ziai WC, Gusdon AM, Hanley DF. Blood pressure in intracerebral haemorrhage: which variables matter? Lancet Neurol. 2019; 18:810–2.

2. Wijdicks EF, Vermeulen M, Murray GD, Hijdra A, van Gijn J. The effects of treating hypertension following aneurysmal subarachnoid hemorrhage. Clin Neurol Neurosurg. 1990; 92:111–7.

3. Kim BJ, Kim JS. Ischemic stroke subtype classification: an asian viewpoint. J Stroke. 2014; 16:8–17.

4. Hong KS, Bang OY, Kim JS, Heo JH, Yu KH, Bae HJ, et al. Stroke statistics in Korea: part II stroke awareness and acute stroke care, a report from the Korean stroke society and clinical research center for stroke. J Stroke. 2013; 15:67–77.

5. Alexander RW. Hypertension and the pathogenesis of atherosclerosis. Oxidative stress and the mediation of arterial inflammatory response: a new perspective. Hypertension. 1995; 25:155–61.
6. Verdecchia P, Angeli F, Reboldi G. Hypertension and atrial fibrillation: doubts and certainties from basic and clinical studies. Circ Res. 2018; 122:352–68.
7. SPS3 Study Group, Benavente OR, Coffey CS, Conwit R, Hart RG, McClure LA, et al. Blood-pressure targets in patients with recent lacunar stroke: the SPS3 randomised trial. Lancet. 2013; 382:507–15.
8. An SJ, Kim TJ, Yoon BW. Epidemiology, risk factors, and clinical features of intracerebral hemorrhage: an update. J Stroke. 2017; 19:3–10.

9. Tikhonoff V, Zhang H, Richart T, Staessen JA. Blood pressure as a prognostic factor after acute stroke. Lancet Neurol. 2009; 8:938–48.

10. Qureshi AI, Ezzeddine MA, Nasar A, Suri MF, Kirmani JF, Hussein HM, et al. Prevalence of elevated blood pressure in 563,704 adult patients with stroke presenting to the ED in the United States. Am J Emerg Med. 2007; 25:32–8.

11. Rodriguez-Luna D, Piñeiro S, Rubiera M, Ribo M, Coscojuela P, Pagola J, et al. Impact of blood pressure changes and course on hematoma growth in acute intracerebral hemorrhage. Eur J Neurol. 2013; 20:1277–83.

12. Butcher KS, Jeerakathil T, Hill M, Demchuk AM, Dowlatshahi D, Coutts SB, et al. The intracerebral hemorrhage acutely decreasing arterial pressure trial. Stroke. 2013; 44:620–6.

13. Anderson CS, Heeley E, Huang Y, Wang J, Stapf C, Delcourt C, et al. Rapid blood-pressure lowering in patients with acute intracerebral hemorrhage. N Engl J Med. 2013; 368:2355–65.

14. Qureshi AI, Palesch YY, Barsan WG, Hanley DF, Hsu CY, Martin RL, et al. Intensive blood-pressure lowering in patients with acute cerebral hemorrhage. N Engl J Med. 2016; 375:1033–43.

15. Hemphill JC 3rd, Greenberg SM, Anderson CS, Becker K, Bendok BR, Cushman M, et al. Guidelines for the management of spontaneous intracerebral hemorrhage: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2015; 46:2032–60.

16. Buletko AB, Thacker T, Cho SM, Mathew J, Thompson NR, Organek N, et al. Cerebral ischemia and deterioration with lower blood pressure target in intracerebral hemorrhage. Neurology. 2018; 91:e1058–66.

17. Arima H, Heeley E, Delcourt C, Hirakawa Y, Wang X, Woodward M, et al. Optimal achieved blood pressure in acute intracerebral hemorrhage: INTERACT2. Neurology. 2015; 84:464–71.

18. Chung PW, Kim JT, Sanossian N, Starkmann S, Hamilton S, Gornbein J, et al. Association between hyperacute stage blood pressure variability and outcome in patients with spontaneous intracerebral hemorrhage. Stroke. 2018; 49:348–54.

19. Divani AA, Liu X, Di Napoli M, Lattanzi S, Ziai W, James ML, et al. Blood pressure variability predicts poor in-hospital outcome in spontaneous intracerebral hemorrhage. Stroke. 2019; 50:2023–9.

20. Connolly ES Jr, Rabinstein AA, Carhuapoma JR, Derdeyn CP, Dion J, Higashida RT, et al. Guidelines for the management of aneurysmal subarachnoid hemorrhage: a guideline for healthcare professionals from the American Heart Association/american Stroke Association. Stroke. 2012; 43:1711–37.
21. Diringer MN, Bleck TP, Claude Hemphill J 3rd, Menon D, Shutter L, Vespa P, et al. Critical care management of patients following aneurysmal subarachnoid hemorrhage: recommendations from the Neurocritical Care Society's Multidisciplinary Consensus Conference. Neurocrit Care. 2011; 15:211–40.

22. Steiner T, Juvela S, Unterberg A, Jung C, Forsting M, Rinkel G, et al. European Stroke Organization guidelines for the management of intracranial aneurysms and subarachnoid haemorrhage. Cerebrovasc Dis. 2013; 35:93–112.

23. Lin QS, Ping-Chen , Lin YX, Lin ZY, Yu LH, Dai LS, et al. Systolic blood pressure variability is a novel risk factor for rebleeding in acute subarachnoid hemorrhage: a case-control study. Medicine (Baltimore). 2016; 95:e3028.
24. Yang M, Pan X, Liang Z, Huang X, Duan M, Cai H, et al. Association between blood pressure variability and the short-term outcome in patients with acute spontaneous subarachnoid hemorrhage. Hypertens Res. 2019; 42:1701–7.

25. Naidech AM, Jovanovic B, Wartenberg KE, Parra A, Ostapkovich N, Connolly ES, et al. Higher hemoglobin is associated with improved outcome after subarachnoid hemorrhage. Crit Care Med. 2007; 35:2383–9.

26. Powers WJ, Rabinstein AA, Ackerson T, Adeoye OM, Bambakidis NC, Becker K, et al. 2018 Guidelines for the early management of patients with acute ischemic stroke: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2018; 49:e46–110.

27. Anderson CS, Huang Y, Lindley RI, Chen X, Arima H, Chen G, et al. Intensive blood pressure reduction with intravenous thrombolysis therapy for acute ischaemic stroke (ENCHANTED): an international, randomised, open-label, blinded-endpoint, phase 3 trial. Lancet. 2019; 393:877–88.
28. Delgado-Mederos R, Ribo M, Rovira A, Rubiera M, Munuera J, Santamarina E, et al. Prognostic significance of blood pressure variability after thrombolysis in acute stroke. Neurology. 2008; 71:552–8.

29. Goyal M, Demchuk AM, Menon BK, Eesa M, Rempel JL, Thornton J, et al. Randomized assessment of rapid endovascular treatment of ischemic stroke. N Engl J Med. 2015; 372:1019–30.
30. Jovin TG, Saver JL, Ribo M, Pereira V, Furlan A, Bonafe A, et al. Diffusion-weighted imaging or computerized tomography perfusion assessment with clinical mismatch in the triage of wake up and late presenting strokes undergoing neurointervention with Trevo (DAWN) trial methods. Int J Stroke. 2017; 12:641–52.

31. Anadani M, Orabi MY, Alawieh A, Goyal N, Alexandrov AV, Petersen N, et al. Blood pressure and outcome after mechanical thrombectomy with successful revascularization. Stroke. 2019; 50:2448–54.

32. Anadani M, Arthur AS, Tsivgoulis G, Simpson KN, Alawieh A, Orabi Y, et al. Blood pressure goals and clinical outcomes after successful endovascular therapy: a multicenter study. Ann Neurol. 2020; 87:830–9.

33. Rasmussen M, Schönenberger S, Hendèn PL, Valentin JB, Espelund US, Sørensen LH, et al. Blood pressure thresholds and neurologic outcomes after endovascular therapy for acute ischemic stroke: an analysis of individual patient data from 3 randomized clinical trials. JAMA Neurol. 2020; 77:622–31.
34. Hong L, Cheng X, Lin L, Bivard A, Ling Y, Butcher K, et al. The blood pressure paradox in acute ischemic stroke. Ann Neurol. 2019; 85:331–9.

35. Leonardi-Bee J, Bath PM, Phillips SJ, Sandercock PA; IST Collaborative Group. Blood pressure and clinical outcomes in the International Stroke Trial. Stroke. 2002; 33:1315–20.

36. Lattanzi S, Silvestrini M, Provinciali L. Elevated blood pressure in the acute phase of stroke and the role of angiotensin receptor blockers. Int J Hypertens. 2013; 2013:941783.

37. Stead LG, Gilmore RM, Vedula KC, Weaver AL, Decker WW, Brown RD Jr. Impact of acute blood pressure variability on ischemic stroke outcome. Neurology. 2006; 66:1878–81.

38. Wahlgren NG, MacMahon DG, De Keyser J, Indredavik B, Ryman T. Intravenous Nimodipine West European Stroke Trial (INWEST) of nimodipine in the treatment of acute ischaemic stroke. Cerebrovasc Dis. 1994; 4:204–10.

39. Horn J, de Haan RJ, Vermeulen M, Limburg M. Very early nimodipine use in stroke (VENUS): a randomized, double-blind, placebo-controlled trial. Stroke. 2001; 32:461–5.
40. Bath PM, Martin RH, Palesch Y, Cotton D, Yusuf S, Sacco R, et al. Effect of telmisartan on functional outcome, recurrence, and blood pressure in patients with acute mild ischemic stroke: a PRoFESS subgroup analysis. Stroke. 2009; 40:3541–6.
41. Sandset EC, Bath PM, Boysen G, Jatuzis D, Kõrv J, Lüders S, et al. The angiotensin-receptor blocker candesartan for treatment of acute stroke (SCAST): a randomised, placebo-controlled, double-blind trial. Lancet. 2011; 377:741–50.

42. Potter JF, Robinson TG, Ford GA, Mistri A, James M, Chernova J, et al. Controlling hypertension and hypotension immediately post-stroke (CHHIPS): a randomised, placebo-controlled, double-blind pilot trial. Lancet Neurol. 2009; 8:48–56.

43. Robinson TG, Potter JF, Ford GA, Bulpitt CJ, Chernova J, Jagger C, et al. Effects of antihypertensive treatment after acute stroke in the Continue or Stop Post-Stroke Antihypertensives Collaborative Study (COSSACS): a prospective, randomised, open, blinded-endpoint trial. Lancet Neurol. 2010; 9:767–75.

44. ENOS Trial Investigators. Efficacy of nitric oxide, with or without continuing antihypertensive treatment, for management of high blood pressure in acute stroke (ENOS): a partial-factorial randomised controlled trial. Lancet. 2015; 385:617–28.
45. Lee M, Ovbiagele B, Hong KS, Wu YL, Lee JE, Rao NM, et al. Effect of blood pressure lowering in early ischemic stroke: meta-analysis. Stroke. 2015; 46:1883–9.
46. Bath PM, Krishnan K. Interventions for deliberately altering blood pressure in acute stroke. Cochrane Database Syst Rev. 2014; 2014:CD000039.

47. Wohlfahrt P, Krajcoviechova A, Jozifova M, Mayer O, Vanek J, Filipovsky J, et al. Low blood pressure during the acute period of ischemic stroke is associated with decreased survival. J Hypertens. 2015; 33:339–45.

48. Vemmos KN, Tsivgoulis G, Spengos K, Zakopoulos N, Synetos A, Manios E, et al. U-shaped relationship between mortality and admission blood pressure in patients with acute stroke. J Intern Med. 2004; 255:257–65.

49. Stead LG, Gilmore RM, Decker WW, Weaver AL, Brown RD Jr. Initial emergency department blood pressure as predictor of survival after acute ischemic stroke. Neurology. 2005; 65:1179–83.

50. He J, Zhang Y, Xu T, Zhao Q, Wang D, Chen CS, et al. Effects of immediate blood pressure reduction on death and major disability in patients with acute ischemic stroke: the CATIS randomized clinical trial. JAMA. 2014; 311:479–89.
51. PATS Collaborating Group. Post-stroke antihypertensive treatment study: a preliminary result. Chin Med J (Engl). 1995; 108:710–7.
52. PROGRESS Collaborative Group. Randomised trial of a perindopril-based blood-pressure-lowering regimen among 6,105 individuals with previous stroke or transient ischaemic attack. Lancet. 2001; 358:1033–41.
53. Yusuf S, Diener HC, Sacco RL, Cotton D, Ounpuu S, Lawton WA, et al. Telmisartan to prevent recurrent stroke and cardiovascular events. N Engl J Med. 2008; 359:1225–37.

54. Liu L, Wang Z, Gong L, Zhang Y, Thijs L, Staessen JA, et al. Blood pressure reduction for the secondary prevention of stroke: a Chinese trial and a systematic review of the literature. Hypertens Res. 2009; 32:1032–40.

55. Ovbiagele B, Diener HC, Yusuf S, Martin RH, Cotton D, Vinisko R, et al. Level of systolic blood pressure within the normal range and risk of recurrent stroke. JAMA. 2011; 306:2137–44.

56. Lawes CM, Bennett DA, Feigin VL, Rodgers A. Blood pressure and stroke: an overview of published reviews. Stroke. 2004; 35:776–85.
57. Powers WJ, Clarke WR, Grubb RL Jr, Videen TO, Adams HP Jr, Derdeyn CP, et al. Lower stroke risk with lower blood pressure in hemodynamic cerebral ischemia. Neurology. 2014; 82:1027–32.

58. Rothwell PM, Howard SC, Spence JD; Carotid Endarterectomy Trialists' Collaboration. Relationship between blood pressure and stroke risk in patients with symptomatic carotid occlusive disease. Stroke. 2003; 34:2583–90.

59. Chaturvedi S, Turan TN, Lynn MJ, Kasner SE, Romano J, Cotsonis G, et al. Risk factor status and vascular events in patients with symptomatic intracranial stenosis. Neurology. 2007; 69:2063–8.

60. Park JH, Ovbiagele B, Hong KS, Kwon SU. Association of systolic blood pressure with progression of symptomatic intracranial atherosclerotic stenosis. J Stroke. 2017; 19:304–11.

61. Park JM, Kim BJ, Kwon SU, Hwang YH, Heo SH, Rha JH, et al. Intensive blood pressure control may not be safe in subacute ischemic stroke by intracranial atherosclerosis: a result of randomized trial. J Hypertens. 2018; 36:1936–41.
62. Kernan WN, Ovbiagele B, Black HR, Bravata DM, Chimowitz MI, Ezekowitz MD, et al. Guidelines for the prevention of stroke in patients with stroke and transient ischemic attack: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2014; 45:2160–236.
Fig. 1.
Blood pressure (BP) management according to treatment of ischemic stroke; (A) intravenous thrombolysis (IV tPA), (B) endovascular treatment, and (C) not indicated for reperfusion therapy and secondary stroke prevention. SBP, systolic blood pressure; ESCAPE, endovascular treatment for small core and anterior circulation proximal occlusion with emphasis on minimizing computed tomography (CT) to recanalization times; DAWN, diffusion-weighted imaging or computerized tomography perfusion assessment with clinical mismatch in the triage of wake up and late presenting strokes undergoing neurointervention.
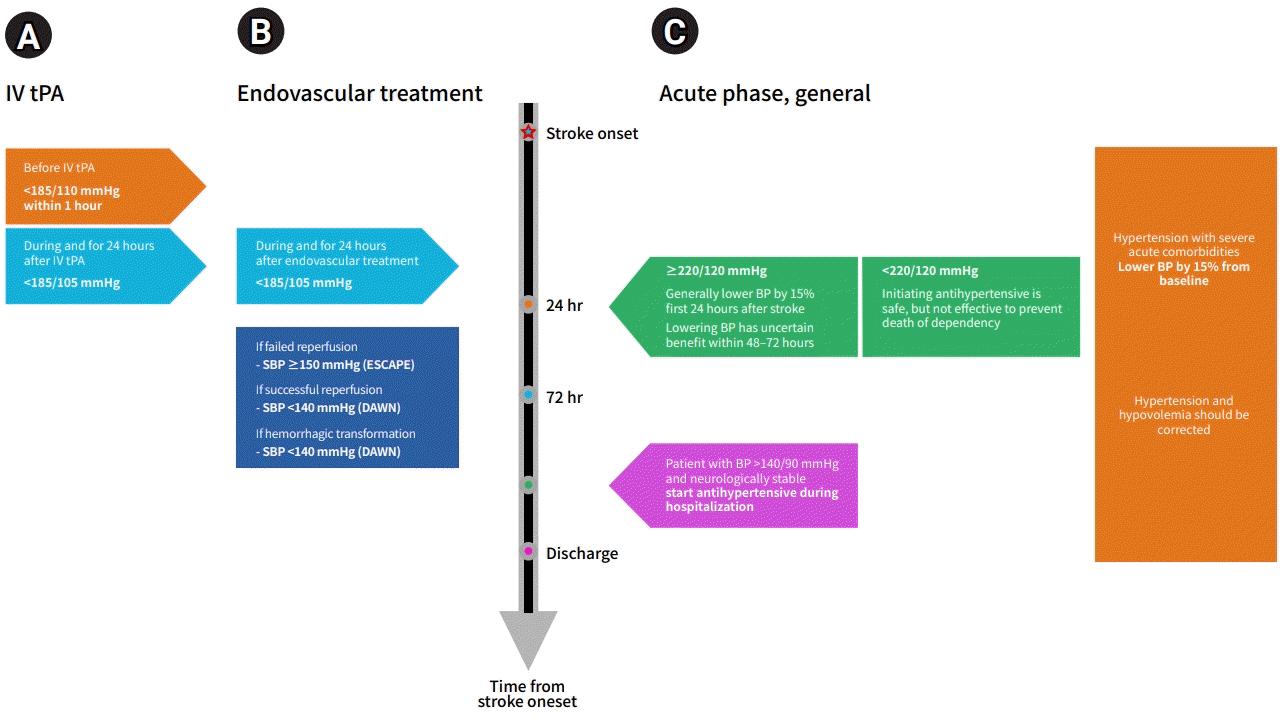
Fig. 2.
Pathophysiology and blood pressure target according to ischemic stroke mechanisms. SBP, systolic blood pressure; WASID, Warfarin Aspirin Symptomatic Intracranial Disease; STABLE-ICAS, strategy for adequate blood pressure lowering in the patients with intracranial atherosclerosis.
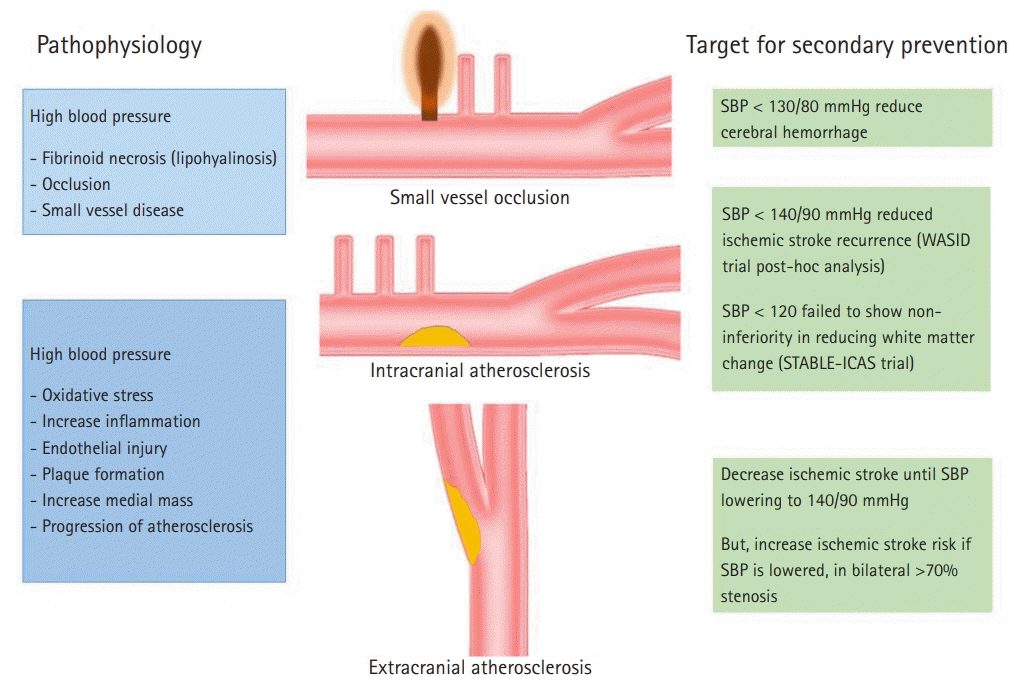
Table 1.
Comparison of INTERACT-2 and ATACH-2 trials
Table 2.
Blood pressure management in hemorrhagic stroke




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download