Abstract
Purpose
This study aimed to evaluate the effect of preoperative tumor staging deviation (PTSD) on the long-term survival of patients undergoing radical gastrectomy for gastric cancer (RGGC).
Materials and Methods
Clinicopathological data of 2,346 patients who underwent RGGC were retrospectively analyzed. The preoperative tumor-lymph node-metastasis (TNM) under-staging group (uTNM) comprised patients who had earlier preoperative TNM than postoperative TNM, and the no preoperative under-staging group (nTNM) comprised the remaining patients.
Results
There were 1,031 uTNM (44.0%) and 1,315 nTNM cases (56.0%). Cox prognostic analysis revealed that PTSD independently affected the overall survival (OS) after surgery. The 5-year OS was lower in the uTNM group (41.8%) than in the nTNM group (71.6%). The patients less than 65 years old, with lower American Society of Anaesthesiologists score, 2–5 cm tumor located at the lower stomach, and cT1 or cN0 preoperative staging would more likely undergo D1+ lymph node dissection (LND) in uTNM (p < 0.05). Logistic analyses revealed that tumor size > 2 cm and body mass index ≤ 22.72 kg/m2 were independent risk factors of preoperative TNM tumor under-staging in patients with cT1N0M0 staging (p < 0.05).
Gastric cancer (GC) is the fifth most common malignancy and the third most common cause of cancer-related deaths worldwide [1]. The treatment strategy for GC has changed over time from a single surgical resection approach to a comprehensive treatment approach based on surgery supplemented with chemotherapy or molecular-targeted drug therapy. As treatment approaches, the tumor staging system for GC is also improved gradually. The latest version of the American Joint Committee on Cancer (AJCC) tumor-lymph node-metastasis (TNM) classification [2] has three independent staging systems for GC: clinical TNM staging (cTNM), pathological TNM staging (pTNM), and neoadjuvant chemotherapy TNM staging (ypTNM), all of which emphasize the importance of different stages in different treatment periods. With the specialization and standardization of a comprehensive treatment plan for GC, correct preoperative staging of GC has become a prerequisite for selecting a reasonable treatment model. Even with surgical treatment, the surgical approaches for different preoperative tumor stages of GC are not the same. Per the Japanese GC Treatment guidelines [3], D1 or D1+ lymph node dissection is performed for early GC without lymph node metastasis, and D2 lymph node dissection is performed for advanced or early GC with lymph node metastasis accessed preoperatively. Some locally advanced GCs are often difficult to treat solely by surgery. In such cases, preoperative treatment such as neoadjuvant chemotherapy is necessary. Furthermore, whether the omentum needs to be resected or the omental sac needs to be removed also depends on preoperative tumor staging. Correct preoperative tumor staging can help clinicians determine the outcome of treatment and the prognosis of their patients. In recent years, preoperative clinical tumor staging of GC has greatly improved because of diagnostic methods such as endoscopic ultrasound (EUS), computed tomography (CT), positron emission tomography (PET)–CT, and laparoscopy, but there are still deviations in clinical tumor staging and pathological tumor staging, which is considered the gold standard. Whether this staging deviation affects the treatment outcome and how to clinically distinguish patients prone to staging deviations are aspects that have not yet been reported. Therefore, this study evaluated the effect of preoperative tumor staging deviations on the long-term survival of patients undergoing radical gastrectomy for GC and explored the intervention measures.
This study retrospectively assessed all patients with GC who underwent radical gastrectomy under the same group of surgeons between June 2007 and November 2013 at Fujian Medical University Union Hospital in China. The following inclusion criteria were set: (1) GC confirmed by preoperative endoscopic biopsy, (2) no distant metastasis or invasion of nearby organs (pancreas, spleen, liver, colon, etc.) found before surgery, and (3) prior radical gastrectomy. The following exclusion criteria were set: (1) preoperative diagnosis of T4b stage or distant metastasis, (2) prior explorative or palliative surgery, (3) chemotherapy before surgery, (4) residual GC, (5) combined organ resection during the operation, (6) postoperative histopathology confirming non-gastric adenocarcinoma, and (7) missing information. Overall, 2,346 patients were included in the study.
All patients underwent routine preoperative examination, including upper gastrointestinal endoscopy and upper gastrointestinal angiography with contrast to confirm the tumor location; chest radiography; CT scanning and ultrasonography (US) of the abdomen to assess preoperative clinical tumor staging; EUS; bone scanning; and PET-CT, if necessary, to assist in assessing preoperative clinical tumor staging.
The preoperative tumor invasion depth (cT) and lymph node metastasis (cN) stage of GC were determined on the basis of preoperative CT imaging results. Distant metastasis (cM) was identified using preoperative CT and US. All preoperative tumor staging results were comprehensively judged by two experienced imaging specialists and experienced surgeons on the basis of the literature and their own experience.
The judgment criteria for cT were based on the criteria of Habermann et al. [4], Hasegawa et al. [5], and Kim et al. [6]. T1 tumors were defined as those that could not be found on images or those with focal thickening of the inner layer with a visible outer layer of the gastric wall and a clear fat plane around the tumor. T2 tumors were defined as those with focal or diffused thickening of the gastric wall with transmural involvement and a smooth outer layer or only a few small linear strands of soft tissue extending into the fat plane involving less than one-third of the tumor extent. T3 tumors were defined as transmural tumors with obvious blurring of at least one-third of the tumor or wide reticular strands surrounding the outer layer of the tumor. T4a tumors were defined as those with obliteration of the fat plane between the gastric tumor and the adjacent organ. T4b tumors were defined as those with invasion of an adjacent organ.
The judgment criteria for cN were based on the criteria of Habermann et al. [4], Lee et al. [7], and Chen et al. [8]. Regional lymph nodes were considered to be metastatic if they were larger than 8 mm in the short-axis diameter; nearly round (longitudinal: transverse diameter ratio < 1.5), showing loss of the normal fatty hilum, or showing marked or heterogeneous enhancement. N0 was defined as non-regional lymph node metastasis. N1 was defined as metastasis in 1–6 regional lymph nodes; N2, in 7–15 regional lymph nodes; and N3, in > 15 regional lymph nodes.
The judgment criteria for cM were as follows: M0 was defined as no distant metastasis and M1, as distant organ metastases, distant lymph node metastasis, or intra-abdominal metastases. Single or multiple halo-enhanced and relatively low-density shadows of parenchymal organs were considered distant organ metastases; para-abdominal aorta, retropancreas, mesenteric root, or other lymph node metastases beyond station 2 metastasis were considered distant lymph node metastases; and ascites and peritoneal thickening or nodular, flaky, and irregular peritoneal thickening were considered intra-abdominal metastases.
According to the 7th AJCC TNM staging classification for patients with GC, tumor staging was evaluated preoperatively and postoperatively. If preoperative tumor staging was earlier than the postoperative tumor staging, it was defined as preoperative tumor under-staging. If the preoperative tumor staging was later than the postoperative tumor staging or the two were consistent, it was defined as the no preoperative tumor under-staging. The TNM, T, and N staging systems for preoperative tumors were similar. In this study, patients with earlier preoperative TNM staging than postoperative TNM staging were classified as the preoperative TNM under-staging group (uTNM group), and the remaining patients were classified as the no preoperative TNM under-staging group (nTNM group).
Lymph node dissection was performed according to the guidelines of the Japanese Gastric Cancer Association [3]. The following lymphadenectomy sequences were performed for distal gastrectomy: No. 6→No. 7, 9, 11p→No. 3, 1→No. 8a, 12a, 5→No. 4sb, and for total gastrectomy: No. 6, 7, 9, 11p→No. 8a, 12a, 5→No. 1→No. 4sb→No. 10, 11d→No. 2. For additional details, please refer to previous publications [9]. The surgeons removed the specimens and divided the lymph nodes into groups according to the Japanese Classification of GC. All specimens were examined and immediately sent to the pathology department. Two or more experienced pathology experts examined each lymph node using palpation without size restriction. All pathological examinations were performed in a standard manner.
The overall follow-up rate was 94.16%, and the median follow-up duration was 72 months (range, 1 to 142 months). Postoperative follow-up was performed in the outpatient department every 3 months for the first 2 years, every 6 months from years 3 to 5, and once a year after 5 years. Most follow-up appointments included a physical examination; laboratory tests, namely, assessment of carbohydrate antigen 19-9 and 72-4 and carcinoembryonic antigen levels; chest radiography; abdominopelvic US or CT; and annual endoscopic examination. Overall survival (OS) was calculated from the day of surgery until death or until the final follow-up date, whichever occurred first.
All statistical analyses were performed using SPSS ver. 25.0 for Windows (IBM Corp., Armonk, NY). All continuous variables are presented as mean±standard deviation. The chi-square or Fisher exact test was used to analyze categorical variables. Cumulative survival rates were compared using the Kaplan-Meier method and log-rank test. The Cox proportional hazards model was used for multivariate prognosis analysis. Logistic regression analysis was carried out to analyze risk factors. Factors with p < 0.05 in univariate analyses were analyzed using multivariate analyses. p < 0.05 were considered significant.
Among the 2,346 patients, the average age was 61.0±11.2 years (range, 12 to 91 years); body mass index (BMI), 22.0±3.0 kg/m2 (range, 13.7 to 37.3 kg/m2); and tumor size, 48.2±26.8 mm (range, 2 to 180 mm). There were 1,031 cases (44.0%) in the uTNM group and 1,315 cases (56.0%) in the nTNM group. Fig. 1 shows the distribution of preoperative staging deviation of the tumor. Table 1 presents the clinicopathological characteristics of all patients.
Prognostic analysis indicated that age, BMI, American Society of Anaesthesiologists (ASA) scores, tumor size, gastrectomy method, preoperative TNM staging assessment deviation, preoperative T staging assessment deviation, preoperative N staging assessment deviation, pathological T staging (pT), pathological N staging (pN), postoperative complications, lymph vascular nerve invasion, primary tumor site, tumor differentiation, adjuvant chemotherapy, Charlson scores, and lymph node noncompliance rates were all prognostic factors on univariate analysis (p < 0.05). Multivariate Cox prognostic analysis revealed that, with the exception of age, BMI, tumor size, pT, pN, gastrectomy method, Charlson scores, and lymph node noncompliance rates independently affected patients’ OS. Preoperative tumor staging assessment deviation also independently affected patients’ overall 5-year survival after surgery (p < 0.05) (Table 2).
The Kaplan-Meier OS survival curve revealed that the OS was significantly lower in the uTNM group than in the nTNM group (5-year OS, 41.8% vs. 71.6%; p < 0.001) (Fig. 2). According to the results of multivariate Cox prognostic analysis, stratified analysis was conducted by the factors which independently affected patients’ OS. Stratified analysis by pT indicated that in patients with pT1 and pT2 there was no significant difference in OS between the two groups, whereas in patients with pT3 and pT4, the OS was significantly lower in the uTNM group than in the nTNM group (p=0.002, p < 0.001) (Fig. 3). Stratified analysis by pN indicated that in patients with pN1, pN2, and pN3, there was no significant difference in the OS between the two groups. In patients with pN0, the OS was significantly lower in the uTNM group than in the nTNM group (p=0.001) (Fig. 4). Stratified analysis by age, BMI, tumor size, gastrectomy method, Charlson scores, and lymph node noncompliance showed that OS was significantly lower in the uTNM group than in the nTNM group (p < 0.001) (S1 Fig.).
In 1,031 cases with preoperative TNM under-staging, the preoperative clinicopathological factors between patients undergoing D1+ and D2 lymph node dissection were compared. The results showed that the patients less than 65 years old, with lower ASA score, 2–5 cm tumor located at the lower stomach, and cT1 or cN0 preoperative staging would more likely undergo D1+ lymph node dissection (p < 0.05) (Table 3).
In patients with cT1N0M0 staging, the univariate and multivariate analyses showed that tumor size > 2 cm and BMI ≤ 22.72 kg/m2 were independent risk factors of preoperative TNM tumor under-staging (p < 0.05) (Table 4).
The prognosis of patients with GC is closely linked to their tumor staging. Thus, accurate preoperative tumor staging of GC is important for guiding treatment-related choices and assessing patient prognosis. In recent years, although the accuracy of clinical staging of GC has greatly increased because of the use of EUS, CT, PET-CT, laparoscopic staging, and other diagnostic methods, the clinical and pathological staging are not always consistent. Several reports have shown that preoperative staging of GC by EUS is inconsistent, especially in terms of depth of invasion. Further, the accuracy of T staging for GC ranged from 41.0% to 86.84% [10–12]. The penetration of ultrasound probes is limited; hence it is difficult to evaluate lymph nodes in distant regions. As a result, the accuracy of N staging for GC was less than that of T staging in EUS [13]. As one of the routine imaging examination techniques, CT plays a key role in patient examination. With the advancement in CT scanning technology and post-processing functions, CT now plays an increasingly important role in the diagnosis, staging, and prognosis evaluation of GC. Previous studies have shown that the accuracy of T and N staging for GC before surgery using CT was not significantly different from that on using EUS [14]. CT for T staging has a high accuracy rate of approximately 73.8%–84.0% [15–17], and for lymph node metastasis, the accuracy is 70%–75% [15–17]. Because CT images can be evaluated more closely than EUS and US images by multiple experts, including surgeons, we are more dependent on preoperative CT images for the assessment of cT and cN at our center. Distant metastases were assessed in combination with findings from other examinations, such as abdominal US.
At present, there are many studies on the importance of accurate staging of GC before surgery, but whether preoperative tumor staging assessment deviation affects patient prognosis has not been reported. This study analyzed the impact of preoperative tumor staging assessment deviation on the prognosis and excluded the influence of other related prognostic factors through stratified analysis. We found that preoperative tumor staging assessment deviation would affect the long-term survival of patients, and patients with preoperative TNM under-staging assessment had a poor prognosis.
Currently, D2 lymph node dissection has gained widespread recognition and is accepted as the standard treatment for advanced or early GCs with lymph node metastasis [18]. The 15-year follow-up results of the Dutch study also showed that D2 lymph node dissection could improve patients’ OS [19]. However, in some cases, D1 or D1+ lymph node dissection will be performed if the preoperative staging is assessed as early GC without lymph node metastasis [3]. In this study, the difference of our action during gastrectomy is degree of lymph node dissection (D1+ vs. D2). What is noteworthy is that preoperative cT1N1-3M0 or cT2-4N0-3M0 staging may be not so important because in both cases the patients will receive D2 dissection. But if the preoperative TNM under-staging occurs in patients with cT1N0M0 staging, the D1+ lymph node dissection would be not enough. Specifically, in case of cT2→pT3, it does not make compliance problem because D2 lymph node dissection would have been performed for this case. However, in case of cT1→pT2, this underestimation may make serious problem because in this case, possibility of doing D1+ lymph node dissection would be high if no enlarged LN is visible in CT scan.
So, the analysis focused on the patients with preoperative TNM under-staging and with cT1N0M0 staging. In the patients with preoperative TNM under-staging, the comparing results between D1+ and D2 lymph node dissection revealed the characteristics of patients more likely undergoing D1+ lymph node dissection. It may suggest that even though the preoperative staging is early in the patients with a young age or lower ASA score, especially with tumor larger than 2 cm and located at the lower stomach, the D2 lymph node dissection could be recommended to be performed, to reduce the inadequate lymph node dissection resulted from preoperative tumor staging deviation.
The study further analysed preoperative predictors of patients with preoperative TNM under-staging in the patients with cT1N0M0 staging. It promoted that in these cases, the tumor size > 2 cm and BMI ≤ 22.72 kg/m2 were independent risk factors of preoperative TNM tumor under-staging. It is possible that in the patients with large tumor, some potential invasion or metastasis cannot be detected by existing imaging technologies. And the fatty tissue in patients with a high BMI, which is of very low density on CT images, can theoretically provide natural contrast to separate tumor and adjacent peritoneum or organs, help to better delineate the tumor [20]. Hence, lack of fat in the patients with a low BMI may increase the difficulty of determination of T-stage on CT. These might lead to preoperative TNM under-staging. So, it reminded that the preoperative staging of cT1N0M0 should be more cautious especially when the patient has tumor size larger than 2 cm or BMI ≤ 22.72 kg/m2. If the inadequate lymph node dissection is carried out, postoperative adjuvant chemotherapy can be used as a remedy method, and the follow-up should be strengthened.
Our study has several limitations. Although this study was a retrospective study with a large cohort, after stratified analysis, the number of cases in each subgroup was unevenly distributed, and some subgroups had relatively smaller cohorts, which might have affected the results of statistical analysis. In addition, since this was a single-center retrospective study, inevitable bias might have been present. Further, a multicentre prospective clinical trial is needed to confirm our results.
In conclusion, it is not rare for surgeons to underestimate tumor staging before surgery, which might cause inadequate lymphatic dissection during surgery and affect the long-term survival of patients undergoing radical gastrectomy for GC. For patients who are prone to tumor under-staging before surgery, such as patients with tumor size larger than 2 cm or BMI ≤ 22.72 kg/m2, full D2 lymph node dissection should be carefully performed during surgery.
Electronic Supplementary Material
Supplementary materials are available at Cancer Research and Treatment website (https://www.e-crt.org).
Notes
Ethical Statement
All patients provided informed consent before surgery. The ethics committee of Fujian Medical University Union Hospital approved this retrospective study (IRB number: 2020KY076).
Author Contributions
Conceived and designed the analysis: Lin M, Chen QY, Huang CM.
Collected the data: Lin M, Xie JW, Wang JB, Lin JX.
Contributed data or analysis tools: Lin M, Zheng CH, Li P, Xie JW, Wang JB, Lin JX, Huang CM.
Performed the analysis: Lin M, Chen QY.
Wrote the paper: Lin M, Chen QY, Huang CM.
Revise the manuscript critically for important intellectual content: Zheng CH, Li P, Huang CM.
ACKNOWLEDGMENTS
This study was funded by Scientific and technological innovation joint capital projects of Fujian Province (2018Y9041). Construction Project of Fujian Province Minimally Invasive Medical Center (No. [2017]171). The second batch of special support funds for Fujian Province innovation and entrepreneurship talents (2016-B013). The general project of sailing fund of Fujian Medical University (2017XQ1026). Fujian provincial health technology project (2018-1-40). China scholarship council (201908350095).
We are thankful to Ru-Hong Tu, Ze-Ning Huang, Ju-Li Lin, Hua-Long Zheng, Guang-Tan Lin, Qing Zhong and Fujian Medical University Union Hospital for managing the GC patient database.
References
1. Ferlay J, Soerjomataram I, Dikshit R, Eser S, Mathers C, Rebelo M, et al. Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer. 2015; 136:E359–86.

2. Amin MB, Greene FL, Edge SB, Compton CC, Gershenwald JE, Brookland RK, et al. The Eighth Edition AJCC Cancer Staging Manual: Continuing to build a bridge from a population-based to a more “personalized” approach to cancer staging. CA Cancer J Clin. 2017; 67:93–9.

3. Japanese Gastric Cancer Association. Japanese gastric cancer treatment guidelines 2010 (ver. 3). Gastric Cancer. 2011; 14:113–23.
4. Habermann CR, Weiss F, Riecken R, Honarpisheh H, Bohnacker S, Staedtler C, et al. Preoperative staging of gastric adenocarcinoma: comparison of helical CT and endoscopic US. Radiology. 2004; 230:465–71.

5. Hasegawa S, Yoshikawa T, Shirai J, Fujikawa H, Cho H, Doiuchi T, et al. A prospective validation study to diagnose serosal invasion and nodal metastases of gastric cancer by multidetector-row CT. Ann Surg Oncol. 2013; 20:2016–22.

6. Kim JW, Shin SS, Heo SH, Lim HS, Lim NY, Park YK, et al. The role of three-dimensional multidetector CT gastrography in the preoperative imaging of stomach cancer: emphasis on detection and localization of the tumor. Korean J Radiol. 2015; 16:80–9.

7. Lee MH, Choi D, Park MJ, Lee MW. Gastric cancer: imaging and staging with MDCT based on the 7th AJCC guidelines. Abdom Imaging. 2012; 37:531–40.

8. Chen CY, Hsu JS, Wu DC, Kang WY, Hsieh JS, Jaw TS, et al. Gastric cancer: preoperative local staging with 3D multi-detector row CT: correlation with surgical and histopathologic results. Radiology. 2007; 242:472–82.
9. Chen QY, Huang CM, Lin JX, Zheng CH, Li P, Xie JW, et al. Laparoscopy-assisted versus open D2 radical gastrectomy for advanced gastric cancer without serosal invasion: a case control study. World J Surg Oncol. 2012; 10:248.

10. Fairweather M, Jajoo K, Sainani N, Bertagnolli MM, Wang J. Accuracy of EUS and CT imaging in preoperative gastric cancer staging. J Surg Oncol. 2015; 111:1016–20.

11. Lei C, Huang L, Wang Y, Huang Y, Huang Y. Comparison of MRI and endoscope ultrasound detection in preoperative T/N staging of gastric cancer. Mol Clin Oncol. 2013; 1:699–702.

12. Giganti F, Orsenigo E, Arcidiacono PG, Nicoletti R, Albarello L, Ambrosi A, et al. Preoperative locoregional staging of gastric cancer: is there a place for magnetic resonance imaging? Prospective comparison with EUS and multidetector computed tomography. Gastric Cancer. 2016; 19:216–25.

13. Cardoso R, Coburn N, Seevaratnam R, Sutradhar R, Lourenco LG, Mahar A, et al. A systematic review and meta-analysis of the utility of EUS for preoperative staging for gastric cancer. Gastric Cancer. 2012; 15(Suppl 1):S19–26.

14. Hwang SW, Lee DH, Lee SH, Park YS, Hwang JH, Kim JW, et al. Preoperative staging of gastric cancer by endoscopic ultrasonography and multidetector-row computed tomography. J Gastroenterol Hepatol. 2010; 25:512–8.

15. Yan C, Zhu ZG, Yan M, Zhang H, Pan ZL, Chen J, et al. Value of multidetector-row computed tomography in the preoperative T and N staging of gastric carcinoma: a large-scale Chinese study. J Surg Oncol. 2009; 100:205–14.

16. Feng XY, Wang W, Luo GY, Wu J, Zhou ZW, Li W, et al. Comparison of endoscopic ultrasonography and multislice spiral computed tomography for the preoperative staging of gastric cancer: results of a single institution study of 610 Chinese patients. PLoS One. 2013; 8:e78846.
17. Kim HJ, Kim AY, Oh ST, Kim JS, Kim KW, Kim PN, et al. Gastric cancer staging at multi-detector row CT gastrography: comparison of transverse and volumetric CT scanning. Radiology. 2005; 236:879–85.

18. Japanese Gastric Cancer Association. Japanese gastric cancer treatment guidelines 2014 (ver. 4). Gastric Cancer. 2017; 20:1–19.
Fig. 1
Distribution of preoperative staging deviation of the tumor. (A) Distribution of preoperative TNM staging deviation of the tumor. (B) Distribution of preoperative T staging deviation of the tumor. (C) Distribution of preoperative N staging deviation of the tumor.
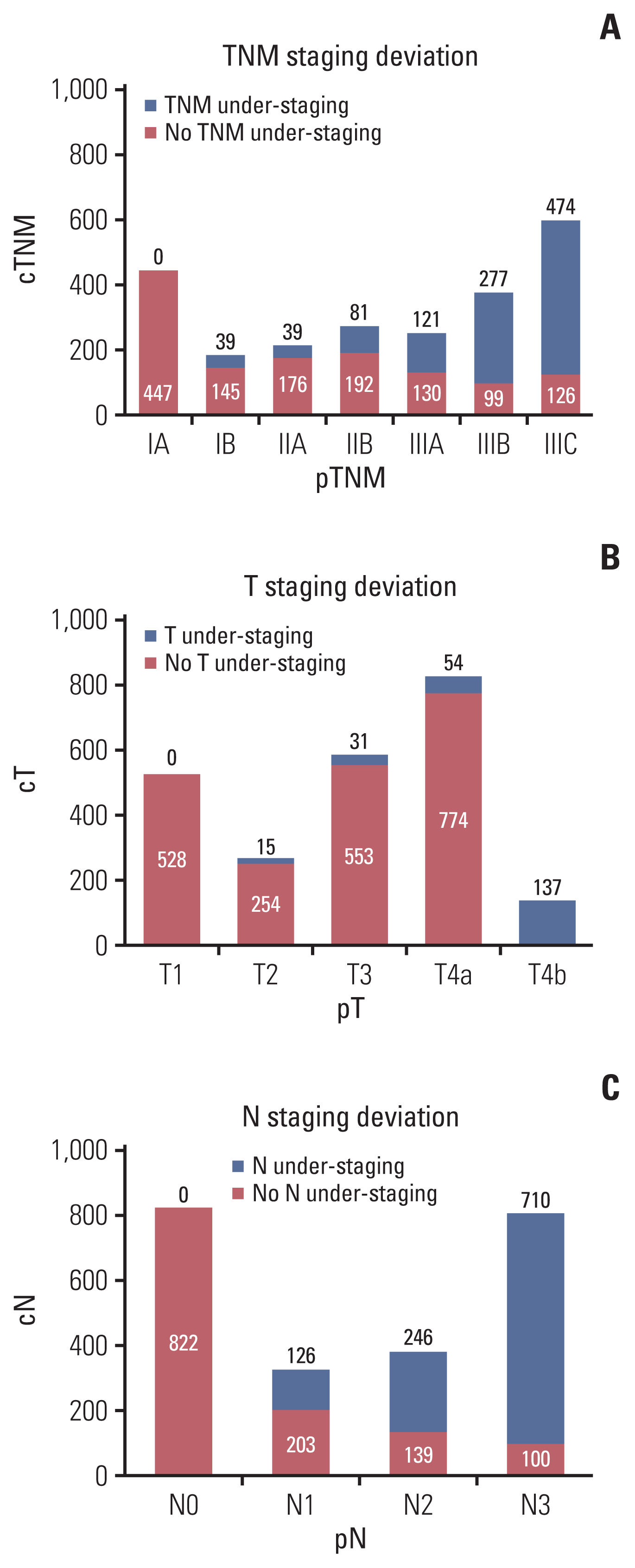
Fig. 2
Overall survival curve of patients with preoperative TNM under-staging and those with no TNM under-staging.
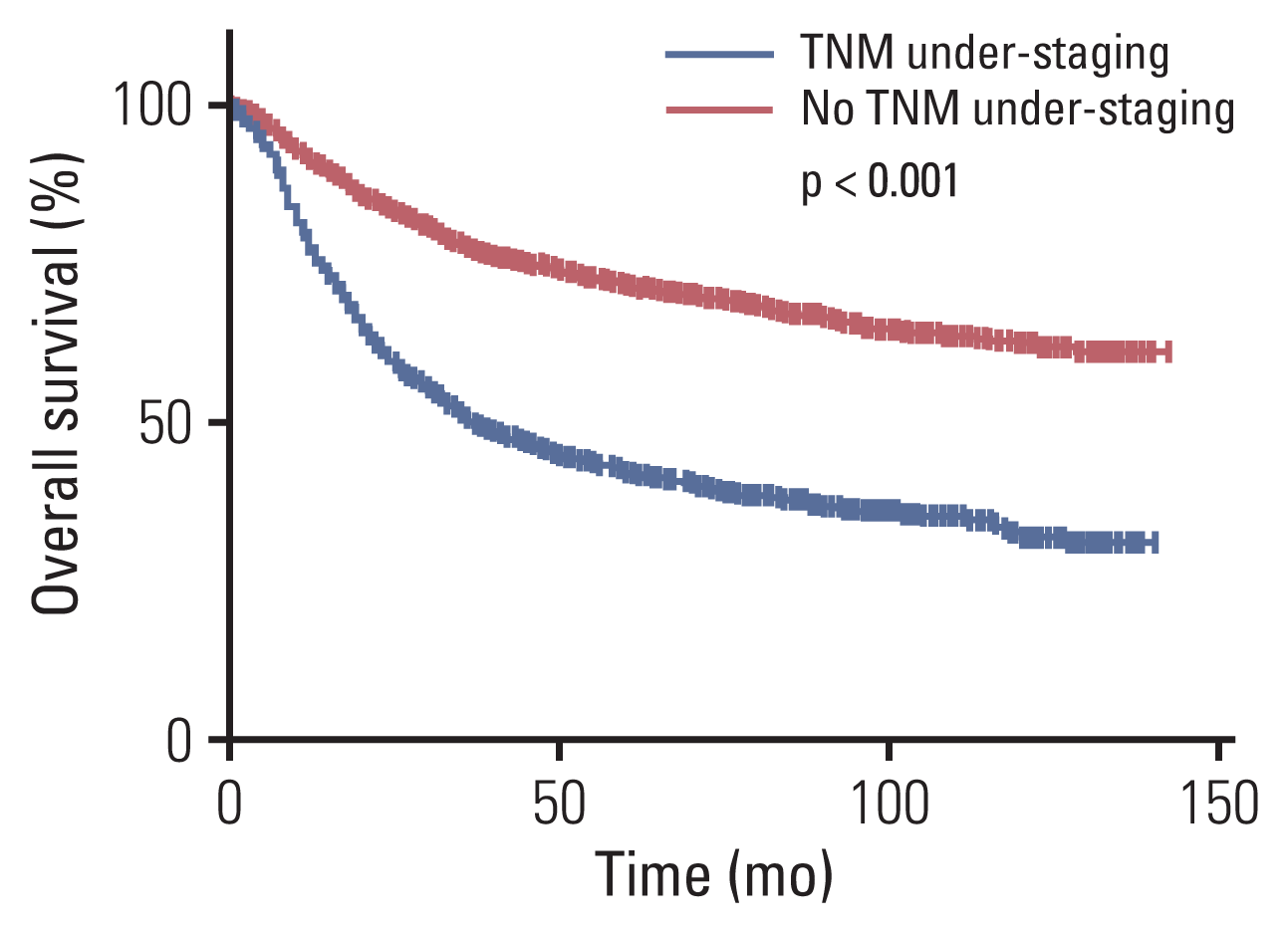
Fig. 3
Overall survival curve of patients with preoperative TNM under-staging and those with no TNM under-staging in the pathological T stratification. (A) Overall survival curve of patients with preoperative TNM under-staging and those with no TNM under-staging among pathological T1 patients. (B) Overall survival curve of patients with preoperative TNM under-staging and those with no TNM under-staging among pathological T2 patients. (C) Overall survival curve of patients with preoperative TNM under-staging and those with no TNM under-staging among pathological T3 patients. (D) Overall survival curve of patients with preoperative TNM under-staging and those with no TNM under-staging among pathological T4 patients.
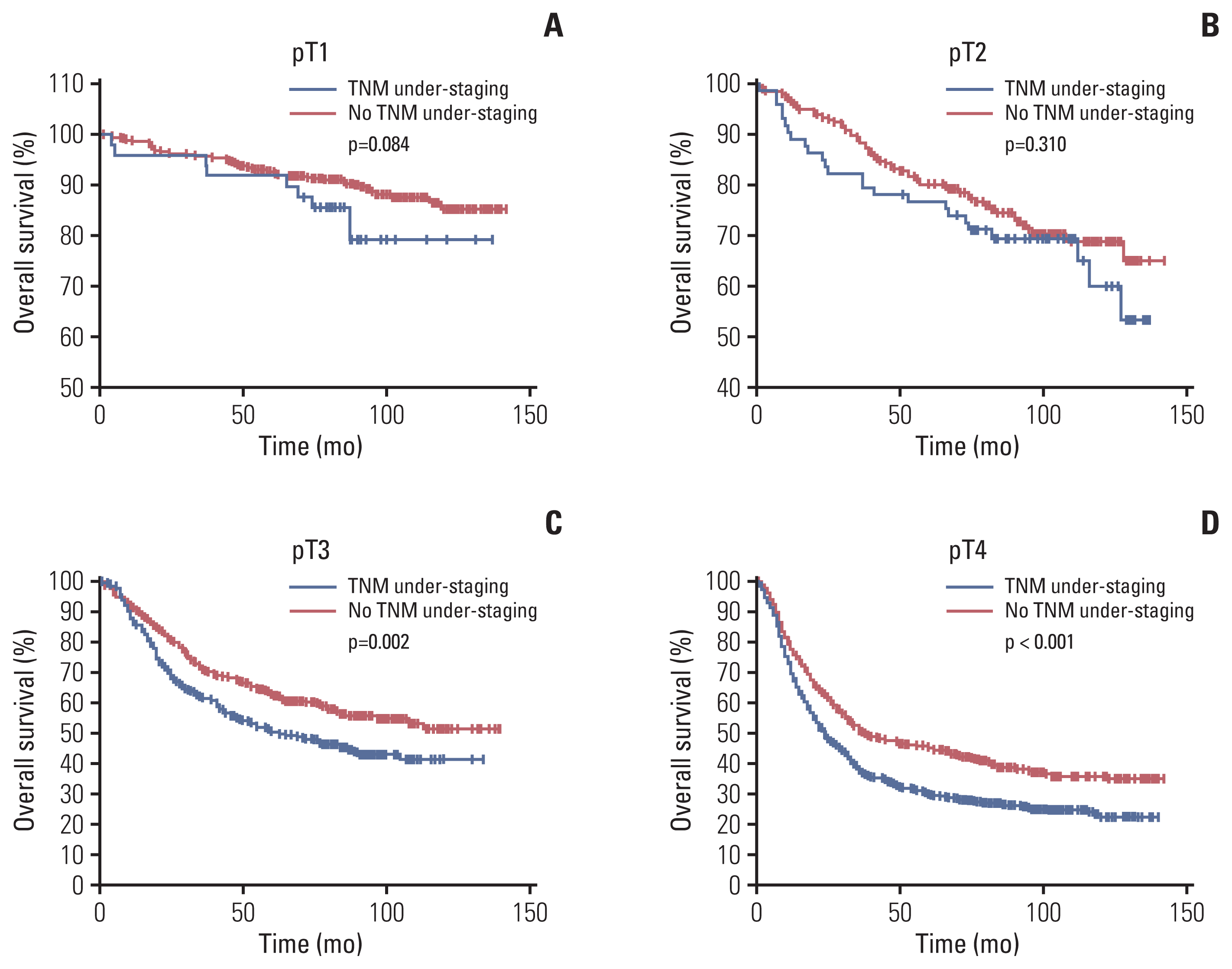
Fig. 4
Overall survival curve of patients with preoperative TNM under-staging and those with no TNM under-staging in the pathological N stratification. (A) Overall survival curve of patients with preoperative TNM under-staging and those with no TNM under-staging among pathological N0 patients. (B) Overall survival curve of patients with preoperative TNM under-staging and those with no TNM under-staging among pathological N1 patients. (C) Overall survival curve of patients with preoperative TNM under-staging and those with no TNM under-staging among pathological N2 patients. (D) Overall survival curve of patients with preoperative TNM under-staging and those with no TNM under-staging among pathological N3 patients.
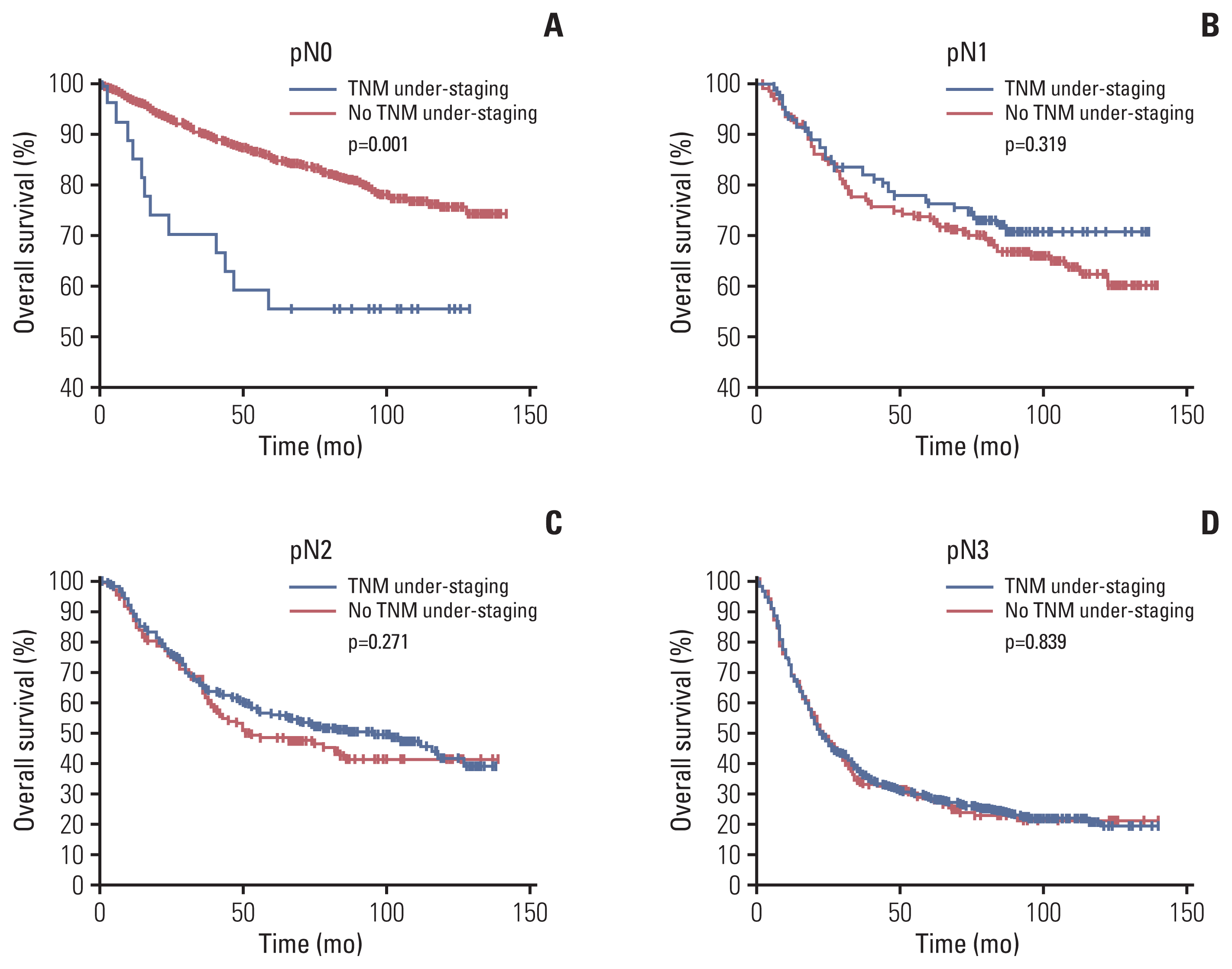
Table 1
Clinicopathological characteristics of all patients
| Characteristic | No. (%) (n=2,346) |
|---|---|
| Sex | |
| Female | 592 (25.2) |
| Male | 1,754 (74.8) |
| Age (yr) | |
| < 65 | 1,444 (61.6) |
| ≥ 65 | 902 (38.4) |
| BMI (kg/m2)a) | |
| ≤ 22.72 | 1,176 (50.1) |
| > 22.72 | 1,170 (49.9) |
| ASA score | |
| I | 1,363 (58.1) |
| II | 858 (36.6) |
| III–IV | 125 (5.3) |
| Charlson score | |
| 0 | 1,596 (68.0) |
| 1–2 | 714 (30.4) |
| 3–5 | 36 (1.5) |
| Previous abdominal surgery | |
| No | 1,990 (84.8) |
| Yes | 356 (15.2) |
| Previous intraperitoneal surgery | |
| No | 2,192 (93.4) |
| Yes | 154 (6.6) |
| cT | |
| cT1 | 528 (22.5) |
| cT2 | 267 (11.4) |
| cT3 | 479 (20.4) |
| cT4 | 1,072 (45.7) |
| cN | |
| cN0 | 1,124 (47.9) |
| cN1 | 573 (24.4) |
| cN2 | 457 (19.5) |
| cN3 | 192 (8.2) |
| cTNM | |
| IA | 437 (18.6) |
| IB | 194 (8.3) |
| IIA | 330 (14.1) |
| IIB | 498 (21.2) |
| IIIA | 425 (18.1) |
| IIIB | 316 (13.5) |
| IIIC | 146 (6.2) |
| Gastrectomy | |
| Total | 1,296 (55.2) |
| Distal | 1,050 (44.8) |
| Size (cm) | |
| < 2 | 232 (9.9) |
| 2–5 | 1,252 (53.4) |
| > 5 | 862 (36.7) |
| Primary site | |
| Lower | 1,052 (44.8) |
| Upper | 420 (17.9) |
| Middle | 596 (25.4) |
| Overlapping lesion of stomach | 278 (11.8) |
| Examined LNs, mean±SD | 31.5±12.9 |
| pT | |
| pT1 | 528 (22.5) |
| pT2 | 269 (11.5) |
| pT3 | 584 (24.9) |
| pT4 | 965 (41.1) |
| pN | |
| N0 | 822 (35.0) |
| N1 | 329 (14.0) |
| N2 | 385 (16.4) |
| N3 | 810 (34.5) |
| pTNM | |
| IA | 447 (19.1) |
| IB | 184 (7.8) |
| IIA | 215 (9.2) |
| IIB | 273 (11.6) |
| IIIA | 251 (10.7) |
| IIIB | 376 (16.0) |
| IIIC | 600 (25.6) |
| Grade | |
| Differentiated | 984 (41.9) |
| Undifferentiated | 1,362 (58.1) |
| Lymph vascular nerve invasion | |
| Negative | 1,853 (79.0) |
| Positive | 493 (21.0) |
| Lymph nodes noncompliance | |
| Noncompliant | 1,140 (48.6) |
| Compliant | 1,206 (51.4) |
| Complications | |
| None | 1,969 (83.9) |
| I–IIb) | 288 (12.3) |
| III–IVb) | 89 (3.8) |
| Adjuvant chemotherapy | |
| No | 1,620 (69.1) |
| Yes | 726 (30.9) |
Table 2
Univariate and multivariate Cox regression models for overall survival analysis of all patients
Table 3
Analysis of clinicopathological factors of different lymph node dissection in patients with preoperative TNM under-staging
| Item | D1+ | D2 | χ2 | p-value |
|---|---|---|---|---|
| Sex | ||||
| Female | 16 (24.6) | 255 (26.4) | 0.100 | 0.752 |
| Male | 49 (75.4) | 711 (73.6) | ||
| Age (yr) | ||||
| < 65 | 47 (72.3) | 576 (59.6) | 4.095 | 0.043 |
| ≥ 65 | 18 (27.7) | 390 (40.4) | ||
| BMI (kg/m2)a) | ||||
| ≤ 22.72 | 43 (66.2) | 638 (66.0) | 0.000 | 0.986 |
| > 22.72 | 22 (33.8) | 328 (34.0) | ||
| ASA score | ||||
| I | 49 (75.4) | 563 (58.3) | 7.768 | 0.021 |
| II | 15 (23.1) | 351 (36.3) | ||
| III–IV | 1 (1.5) | 52 (5.4) | ||
| Charlson score | ||||
| 0 | 51 (78.5) | 667 (69.0) | 3.200 | 0.202 |
| 1–2 | 14 (21.5) | 281 (29.1) | ||
| 3–5 | 0 | 18 (1.9) | ||
| Previous abdominal surgery | ||||
| No | 59 (90.6) | 825 (85.4) | 1.434 | 0.231 |
| Yes | 6 (9.2) | 141 (14.6) | ||
| Previous intraperitoneal surgery | ||||
| No | 58 (89.2) | 910 (94.2) | 2.624 | 0.105 |
| Yes | 7 (10.8) | 56 (5.8) | ||
| cT | ||||
| cT1 | 65 (100) | 31 (3.2) | 89.923 | < 0.001 |
| cT2 | 0 | 81 (8.4) | ||
| cT3 | 0 | 252 (26.1) | ||
| cT4 | 0 | 602 (62.3) | ||
| cN | ||||
| cN0 | 65 (100) | 384 (39.7) | 89.923 | < 0.001 |
| cN1 | 0 | 336 (34.8) | ||
| cN2 | 0 | 242 (25.1) | ||
| cN3 | 0 | 4 (0.4) | ||
| cTNM | ||||
| IA | 65 (100) | 0 | 1,031.000 | < 0.001 |
| IB | 0 | 58 (6.0) | ||
| IIA | 0 | 169 (17.5) | ||
| IIB | 0 | 309 (32.0) | ||
| IIIA | 0 | 250 (25.9) | ||
| IIIB | 0 | 180 (18.6) | ||
| Size (cm) | ||||
| < 2 | 12 (18.5) | 15 (1.5) | 101.176 | < 0.001 |
| 2–5 | 48 (73.8) | 443 (45.9) | ||
| > 5 | 5 (7.7) | 508 (52.6) | ||
| Primary site | ||||
| Lower | 38 (58.5) | 404 (41.8) | 12.939 | 0.005 |
| Upper | 3 (4.6) | 204 (21.1) | ||
| Middle | 17 (26.1) | 222 (23.0) | ||
| Overlapping lesion of stomach | 7 (10.8) | 136 (14.1) | ||
Table 4
Univariate and multivariate analyses of the influence of preoperative TNM under-staging in patients with cT1N0M0 staging
| Variable | Univariate model | Full multivariate model | ||||
|---|---|---|---|---|---|---|
|
|
|
|||||
| OR | 95% CI | p-value | OR | 95% CI | p-value | |
| Sex | ||||||
|
|
||||||
| Female | Reference | 0.911 | - | - | ||
|
|
||||||
| Male | 1.036 | 0.562–1.907 | 0.911 | - | - | - |
|
|
||||||
| Age (yr) | ||||||
|
|
||||||
| < 65 | Reference | 0.441 | - | - | ||
|
|
||||||
| ≥ 65 | 0.794 | 0.443–1.426 | 0.441 | - | - | - |
|
|
||||||
| BMI (kg/m2)a) | ||||||
|
|
||||||
| ≤ 22.72 | Reference | 0.032 | Reference | 0.042 | ||
|
|
||||||
| > 22.72 | 0.546 | 0.314–0.948 | 0.032 | 0.559 | 0.319–0.980 | 0.042 |
|
|
||||||
| ASA score | ||||||
|
|
||||||
| 1 | Reference | 0.147 | - | - | ||
|
|
||||||
| 2 | 0.578 | 0.311–1.072 | 0.082 | - | - | - |
|
|
||||||
| 3 | 0.341 | 0.044–2.655 | 0.304 | - | - | - |
|
|
||||||
| Previous abdominal surgery | ||||||
|
|
||||||
| No | Reference | 0.172 | - | - | ||
|
|
||||||
| Yes | 0.540 | 0.223–1.307 | 0.172 | - | - | - |
|
|
||||||
| Previous intraperitoneal surgery | ||||||
|
|
||||||
| No | Reference | 0.126 | - | - | ||
|
|
||||||
| Yes | 2.017 | 0.821–4.959 | 0.126 | - | - | - |
|
|
||||||
| Comorbidity | ||||||
|
|
||||||
| No | Reference | 0.080 | - | - | ||
|
|
||||||
| Yes | 0.569 | 0.303–1.069 | 0.080 | - | - | - |
|
|
||||||
| Charlson score | ||||||
|
|
||||||
| 0 | Reference | 0.258 | - | - | ||
|
|
||||||
| 1–2 | 0.589 | 0.313–1.107 | 0.258 | - | - | - |
|
|
||||||
| Size (cm) | ||||||
|
|
||||||
| < 2 | Reference | 0.003 | Reference | 0.003 | ||
|
|
||||||
| 2–5 | 2.779 | 1.427–5.412 | 0.003 | 2.705 | 1.385–5.280 | 0.004 |
|
|
||||||
| > 5 | 5.606 | 1.672–18.796 | 0.005 | 5.718 | 1.689–19.357 | 0.005 |
|
|
||||||
| Primary site | ||||||
|
|
||||||
| Distal third | Reference | 0.092 | - | - | ||
|
|
||||||
| Mid third | 0.365 | 0.108–1.229 | 0.104 | - | - | - |
|
|
||||||
| Proximal third | 1.640 | 0.868–3.099 | 0.127 | - | - | - |
|
|
||||||
| Overlapping lesion of stomach | 1.520 | 0.620–3.725 | 0.360 | - | - | - |




 PDF
PDF Citation
Citation Print
Print


 XML Download
XML Download