1. Balakrishnan M, George R, Sharma A, Graham DY. Changing trends in stomach cancer throughout the world. Curr Gastroenterol Rep. 2017; 19:36.

2. Jung KW, Won YJ, Kong HJ, Lee ES. Cancer statistics in Korea: incidence, mortality, survival, and prevalence in 2016. Cancer Res Treat. 2019; 51:417–30.

4. IARC Working Group on the Evaluation of Carcinogenic Risk to Humans. Schistosomes, liver flukes and Helicobacter pylori. IARC Monographs on the Evaluation of Carcinogenic Risks to Humans, No. 61 [Internet]. Lyon: International Agency for Research on Cancer;1994. [cited 2019 Dec 20]. Available from:
https://www.ncbi.nlm.nih.gov/books/NBK487782/
.
5. Ko KP. Epidemiology of gastric cancer in Korea. J Korean Med Assoc. 2019; 62:398–406.

6. Lim SH, Kwon JW, Kim N, Kim GH, Kang JM, Park MJ, et al. Prevalence and risk factors of Helicobacter pylori infection in Korea: nationwide multicenter study over 13 years. BMC Gastroenterol. 2013; 13:104.

7. Kim N, Kim JJ, Choe YH, Kim HS, Kim JI, Chung IS, et al. Diagnosis and treatment guidelines for Helicobacter pylori infection in Korea. Korean J Gastroenterol. 2009; 54:269–78.

8. Brooks-Pollock E, Danon L. Defining the population attributable fraction for infectious diseases. Int J Epidemiol. 2017; 46:976–82.

9. Hanley JA. A heuristic approach to the formulas for population attributable fraction. J Epidemiol Community Health. 2001; 55:508–14.

10. Levin ML. The occurrence of lung cancer in man. Acta Unio Int Contra Cancrum. 1953; 9:531–41.
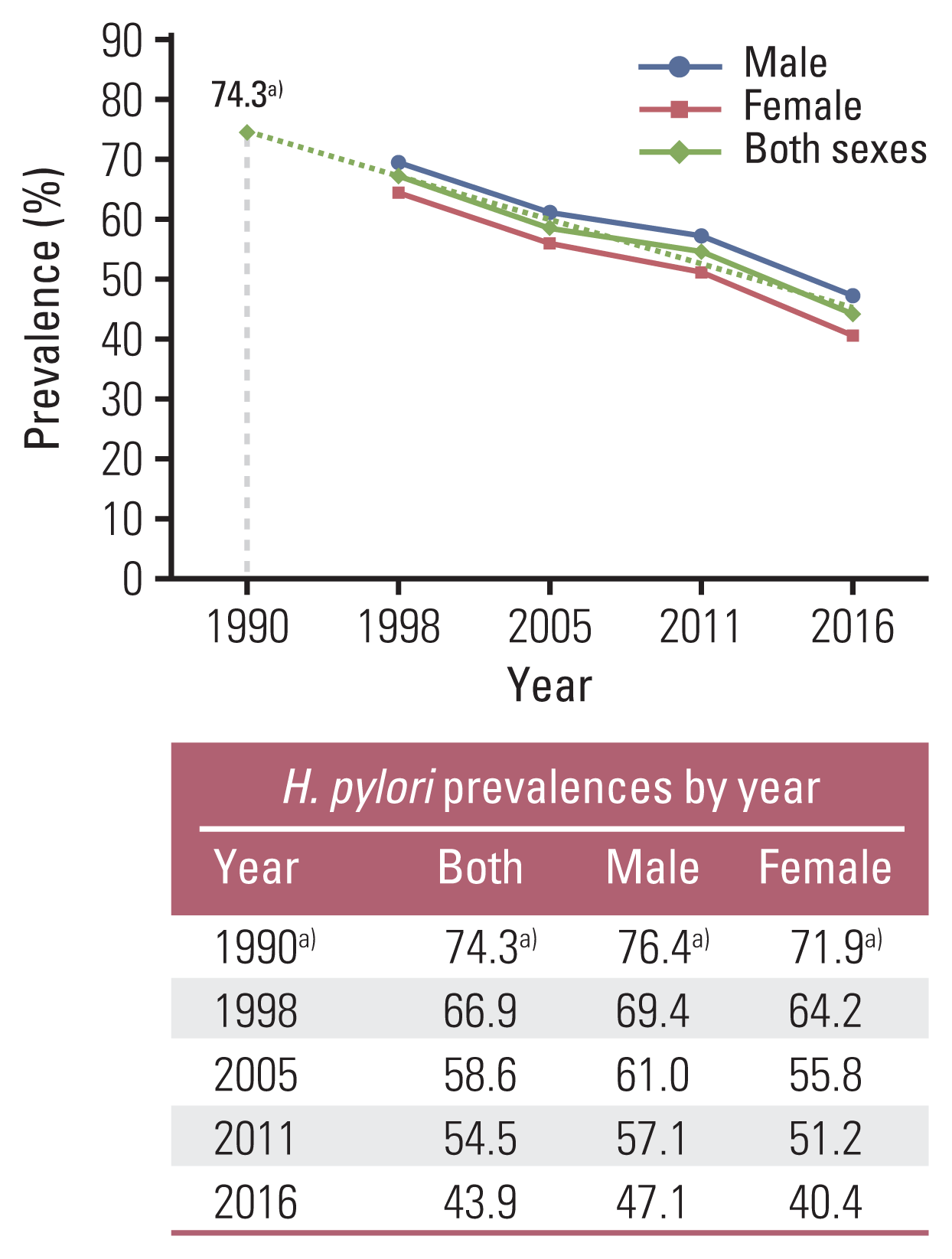
11. Lim SH, Kim N, Kwon JW, Kim SE, Baik GH, Lee JY, et al. Trends in the seroprevalence of Helicobacter pylori infection and its putative eradication rate over 18 years in Korea: a cross-sectional nationwide multicenter study. PLoS One. 2018; 13:e0204762.

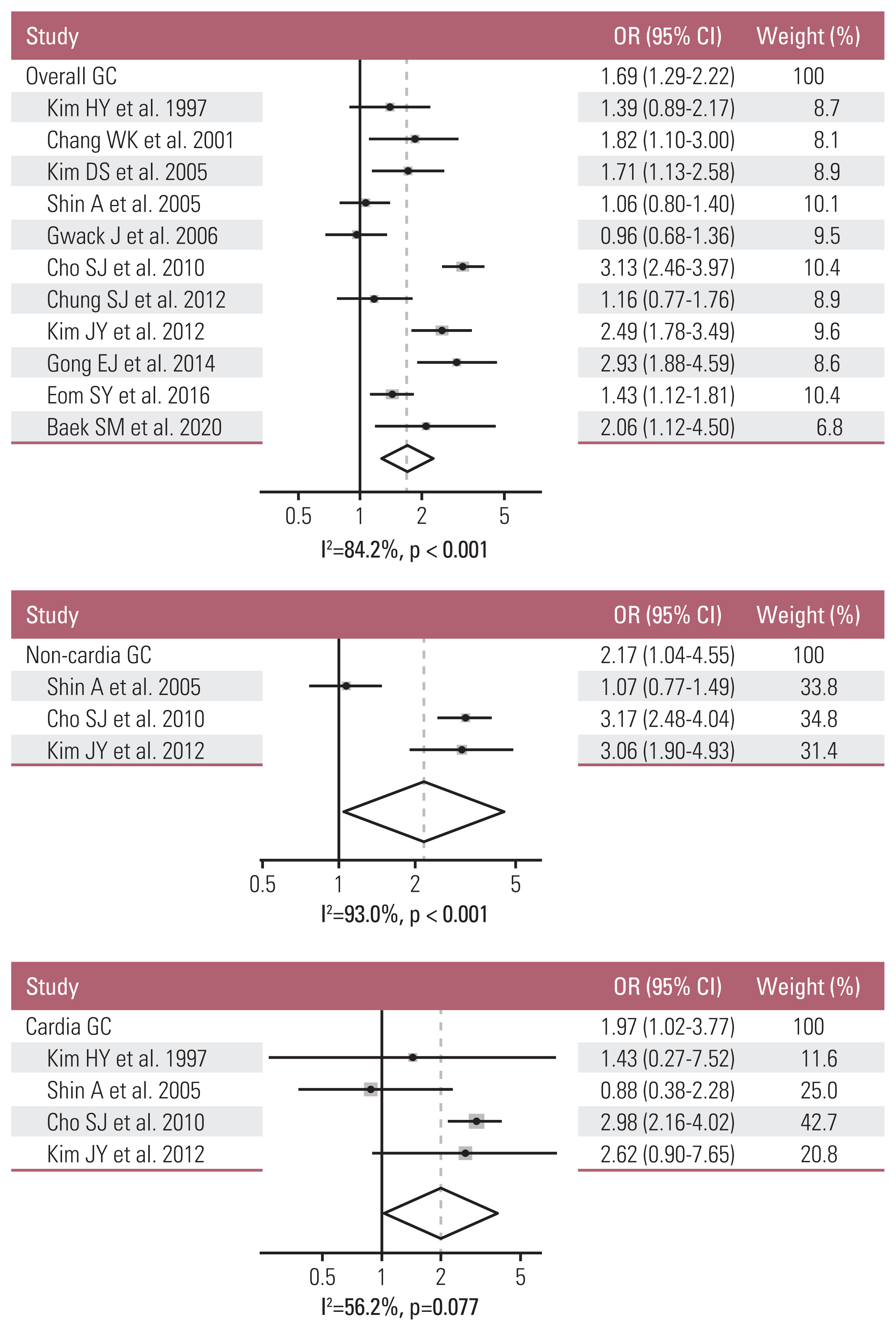
12. Bae JM, Kim EH. Helicobacter pylori infection and risk of gastric cancer in Korea: a quantitative systematic review. J Prev Med Public Health. 2016; 49:197–204.
13. Kim HY, Cho BD, Chang WK, Kim DJ, Kim YB, Park CK, et al. Helicobacter pylori infection and the risk of gastric cancer among the Korean population. J Gastroenterol Hepatol. 1997; 12:100–3.

14. Chang WK, Kim HY, Kim DJ, Lee J, Park CK, Yoo JY, et al. Association between Helicobacter pylori infection and the risk of gastric cancer in the Korean population: prospective case-controlled study. J Gastroenterol. 2001; 36:816–22.

15. Kim DS, Lee MS, Kim YS, Kim DH, Bae JM, Shin MH, et al. Effect modification by vitamin C on the relation between gastric cancer and Helicobacter pylori. Eur J Epidemiol. 2005; 20:67–71.

16. Shin A, Shin HR, Kang D, Park SK, Kim CS, Yoo KY. A nested case-control study of the association of Helicobacter pylori infection with gastric adenocarcinoma in Korea. Br J Cancer. 2005; 92:1273–5.

17. Gwack J, Shin A, Kim CS, Ko KP, Kim Y, Jun JK, et al. CagA-producing Helicobacter pylori and increased risk of gastric cancer: a nested case-control study in Korea. Br J Cancer. 2006; 95:639–41.

18. Cho SJ, Choi IJ, Kim CG, Lee JY, Kook MC, Seong MW, et al. Helicobacter pylori seropositivity is associated with gastric cancer regardless of tumor subtype in Korea. Gut Liver. 2010; 4:466–74.

19. Chung SJ, Park MJ, Kang SJ, Kang HY, Chung GE, Kim SG, et al. Effect of annual endoscopic screening on clinicopathologic characteristics and treatment modality of gastric cancer in a high-incidence region of Korea. Int J Cancer. 2012; 131:2376–84.

20. Kim JY, Lee HS, Kim N, Shin CM, Lee SH, Park YS, et al. Prevalence and clinicopathologic characteristics of gastric cardia cancer in South Korea. Helicobacter. 2012; 17:358–68.

21. Gong EJ, Ahn JY, Jung HY, Lim H, Choi KS, Lee JH, et al. Risk factors and clinical outcomes of gastric cancer identified by screening endoscopy: a case-control study. J Gastroenterol Hepatol. 2014; 29:301–9.

22. Eom SY, Hong SM, Yim DH, Kwon HJ, Kim DH, Yun HY, et al. Additive interactions between PRKAA1 polymorphisms and Helicobacter pylori CagA infection associated with gastric cancer risk in Koreans. Cancer Med. 2016; 5:3236–335.
23. Baek SM, Kim N, Kwon YJ, Lee HS, Kim HY, Lee J, et al. Role of serum pepsinogen II and Helicobacter pylori status in the detection of diffuse-type early gastric cancer in young individuals in South Korea. Gut Liver. 2020; 14:439–49.

24. Higgins JP, Thompson SG. Quantifying heterogeneity in a meta-analysis. Stat Med. 2002; 21:1539–58.

25. Lee SH, Kim MC, Jeon SW, Lee KN, Park JJ, Hong SJ. Risk factors and clinical outcomes of non-curative resection in patients with early gastric cancer treated with endoscopic submucosal dissection: a retrospective multicenter study in Korea. Clin Endosc. 2020; 53:196–205.

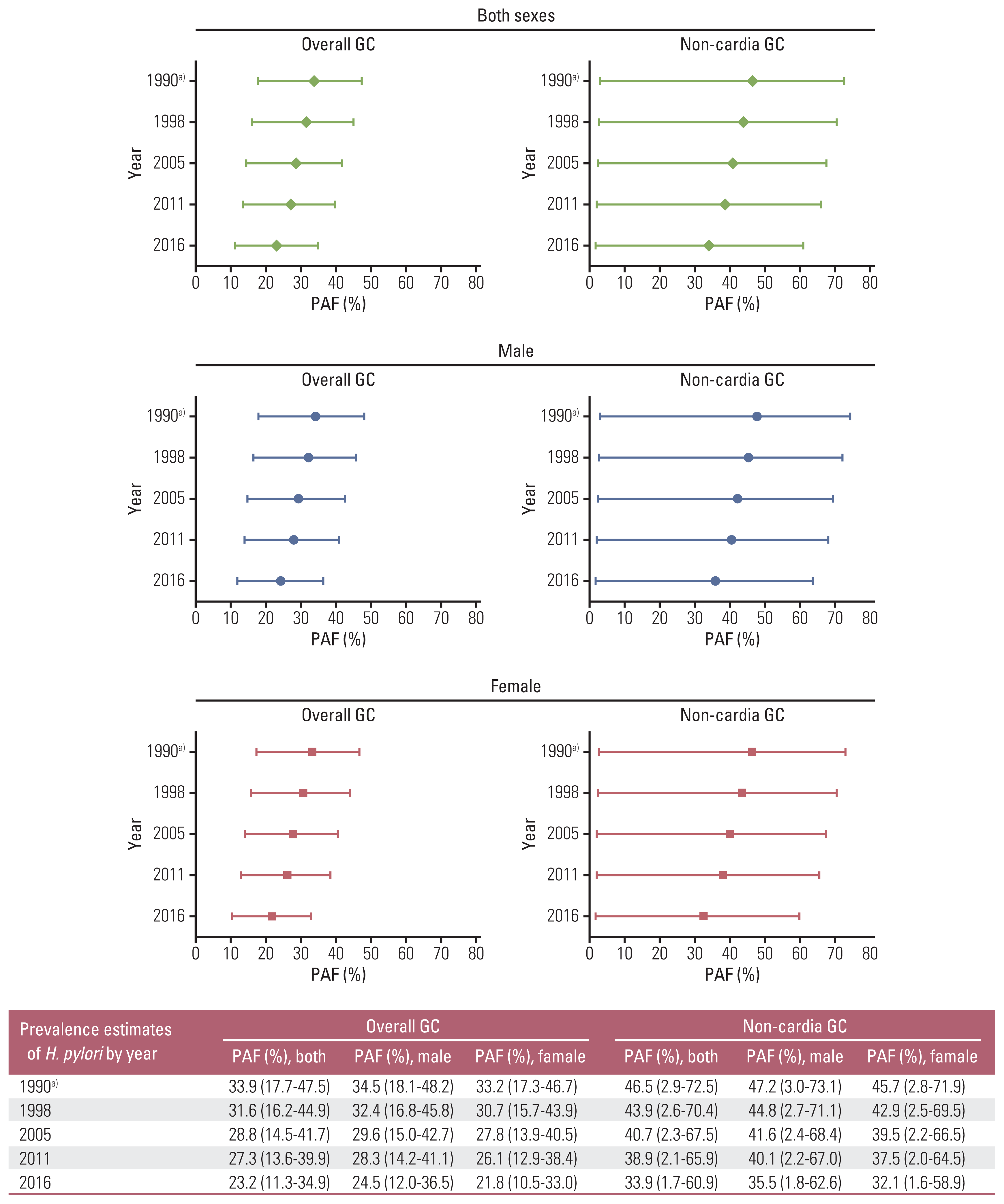
26. Shin A, Park S, Shin HR, Park EH, Park SK, Oh JK, et al. Population attributable fraction of infection-related cancers in Korea. Ann Oncol. 2011; 22:1435–42.

27. Ko KP, Shin A, Cho S, Park SK, Yoo KY. Environmental contributions to gastrointestinal and liver cancer in the Asia-Pacific region. J Gastroenterol Hepatol. 2018; 33:111–20.

28. Helicobacter and Cancer Collaborative Group. Gastric cancer and Helicobacter pylori: a combined analysis of 12 case control studies nested within prospective cohorts. Gut. 2001; 49:347–53.
29. Jiang J, Chen Y, Shi J, Song C, Zhang J, Wang K. Population attributable burden of Helicobacter pylori-related gastric cancer, coronary heart disease, and ischemic stroke in China. Eur J Clin Microbiol Infect Dis. 2017; 36:199–212.

30. Shikata K, Doi Y, Yonemoto K, Arima H, Ninomiya T, Kubo M, et al. Population-based prospective study of the combined influence of cigarette smoking and Helicobacter pylori infection on gastric cancer incidence: the Hisayama Study. Am J Epidemiol. 2008; 168:1409–15.

31. Kim N. Chemoprevention of gastric cancer by Helicobacter pylori eradication and its underlying mechanism. J Gastroenterol Hepatol. 2019; 34:1287–95.
32. Oh S, Kim N, Kwon JW, Shin CM, Choi YJ, Lee DH, et al. Effect of Helicobacter pylori eradication and ABO genotype on gastric cancer development. Helicobacter. 2016; 21:596–605.
33. Choi JM, Kim SG, Choi J, Park JY, Oh S, Yang HJ, et al. Effects of Helicobacter pylori eradication for metachronous gastric cancer prevention: a randomized controlled trial. Gastrointest Endosc. 2018; 88:475–85.

34. Choi IJ, Kook MC, Kim YI, Cho SJ, Lee JY, Kim CG, et al. Helicobacter pylori therapy for the prevention of metachronous gastric cancer. N Engl J Med. 2018; 378:1085–95.

35. Malfertheiner P. Helicobacter pylori treatment for gastric cancer prevention. N Engl J Med. 2018; 378:1154–6.

36. Fock KM, Ang TL. Epidemiology of Helicobacter pylori infection and gastric cancer in Asia. J Gastroenterol Hepatol. 2010; 25:479–86.
37. Jagerstad M, Skog K. Genotoxicity of heat-processed foods. Mutat Res. 2005; 574:156–72.
38. D’Elia L, Rossi G, Ippolito R, Cappuccio FP, Strazzullo P. Habitual salt intake and risk of gastric cancer: a meta-analysis of prospective studies. Clin Nutr. 2012; 31:489–98.

39. Kim J, Park S, Nam BH. Gastric cancer and salt preference: a population-based cohort study in Korea. Am J Clin Nutr. 2010; 91:1289–93.

40. Yang S, Lee J, Choi IJ, Kim YW, Ryu KW, Sung J, et al. Effects of alcohol consumption, ALDH2 rs671 polymorphism, and Helicobacter pylori infection on the gastric cancer risk in a Korean population. Oncotarget. 2017; 8:6630–41.

41. Butt J, Varga MG, Wang T, Tsugane S, Shimazu T, Zheng W, et al. Smoking, Helicobacter pylori serology, and gastric cancer risk in prospective studies from China, Japan, and Korea. Cancer Prev Res (Phila). 2019; 12:667–74.





 PDF
PDF Citation
Citation Print
Print


 XML Download
XML Download