1. Ferlay J, Parkin DM, Steliarova-Foucher E. Estimates of cancer incidence and mortality in Europe in 2008. Eur J Cancer. 2010; 46:765–81.

2. Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D. Global cancer statistics. CA Cancer J Clin. 2011; 61:69–90.

3. Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018; 68:394–424.

4. Sorosky JI. Endometrial cancer. Obstet Gynecol. 2012; 120:383–97.

5. Constantine GD, Kessler G, Graham S, Goldstein SR. Increased incidence of endometrial cancer following the Women’s Health Initiative: an assessment of risk factors. J Womens Health (Larchmt). 2019; 28:237–43.

6. Avgerinos KI, Spyrou N, Mantzoros CS, Dalamaga M. Obesity and cancer risk: emerging biological mechanisms and perspectives. Metabolism. 2019; 92:121–35.

7. Linkov F, Goughnour SL, Adambekov S, Edwards RP, Donnellan N, Bovbjerg DH. Lifestyle interventions to reduce the risk of obesity-associated gynecologic malignancies: a focus on endometrial cancer. Berger M, Klopp A, Lu K, editors. Focus on gynecologic malignancies. Energy balance and cancer. 13. Cham: Springer;2018. p. 137–65.

8. Gierach GL, Chang SC, Brinton LA, Lacey JV Jr, Hollenbeck AR, Schatzkin A, et al. Physical activity, sedentary behavior, and endometrial cancer risk in the NIH-AARP Diet and Health Study. Int J Cancer. 2009; 124:2139–47.

9. Kawachi A, Shimazu T, Budhathoki S, Sawada N, Yamaji T, Iwasaki M, et al. Association of BMI and height with the risk of endometrial cancer, overall and by histological subtype: a population-based prospective cohort study in Japan. Eur J Cancer Prev. 2019; 28:196–202.

10. Dunneram Y, Greenwood DC, Cade JE. Diet, menopause and the risk of ovarian, endometrial and breast cancer. Proc Nutr Soc. 2019; 78:438–48.

11. Pronk NP, Anderson LH, Crain AL, Martinson BC, O’Connor PJ, Sherwood NE, et al. Meeting recommendations for multiple healthy lifestyle factors: prevalence, clustering, and predictors among adolescent, adult, and senior health plan members. Am J Prev Med. 2004; 27:25–33.
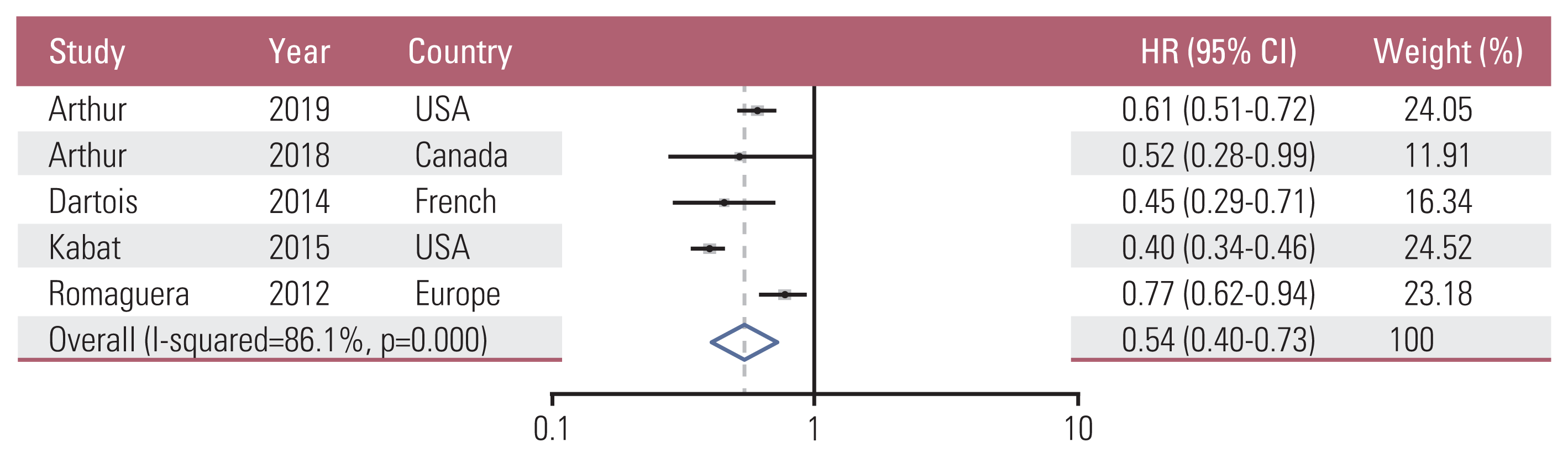
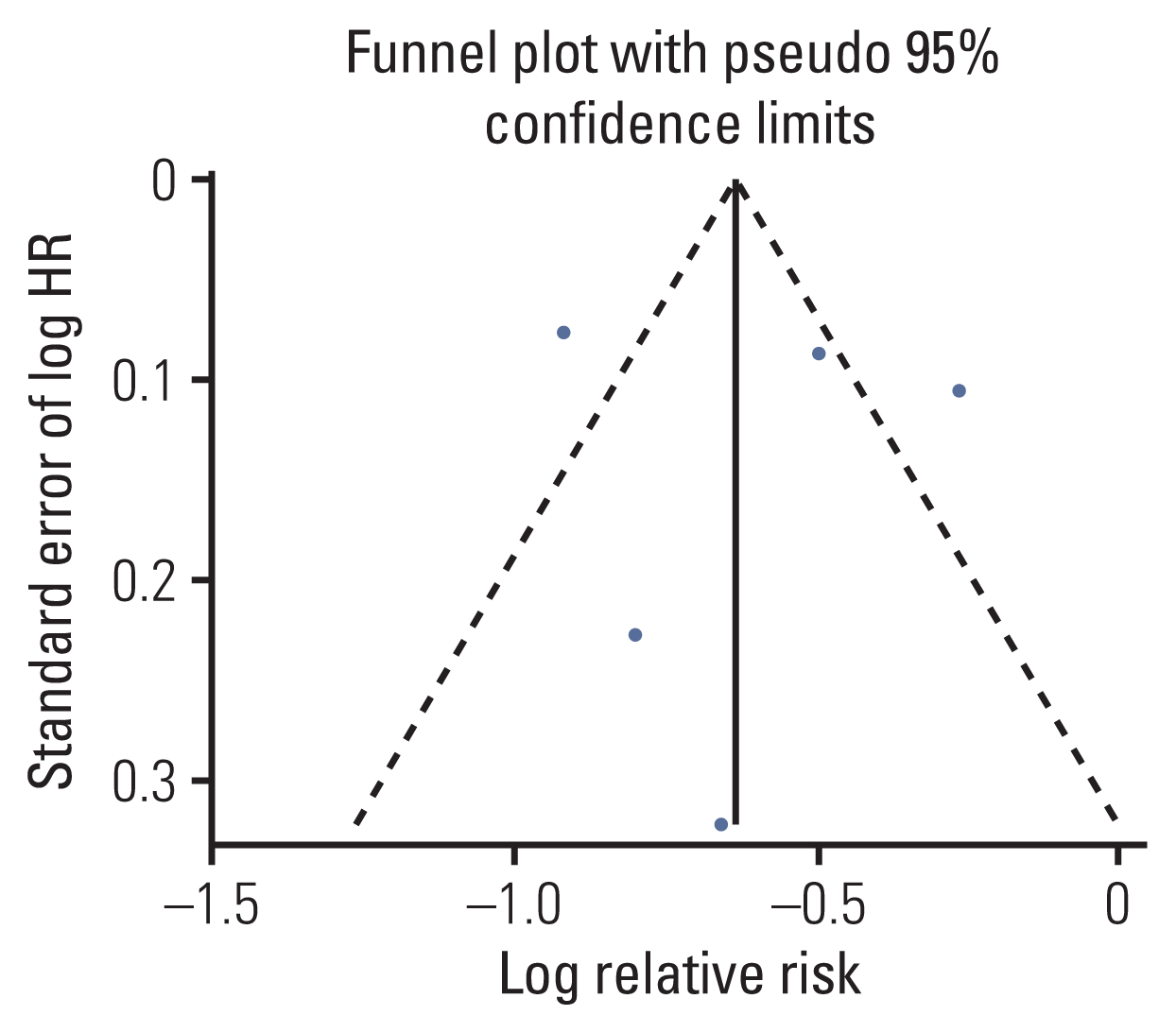
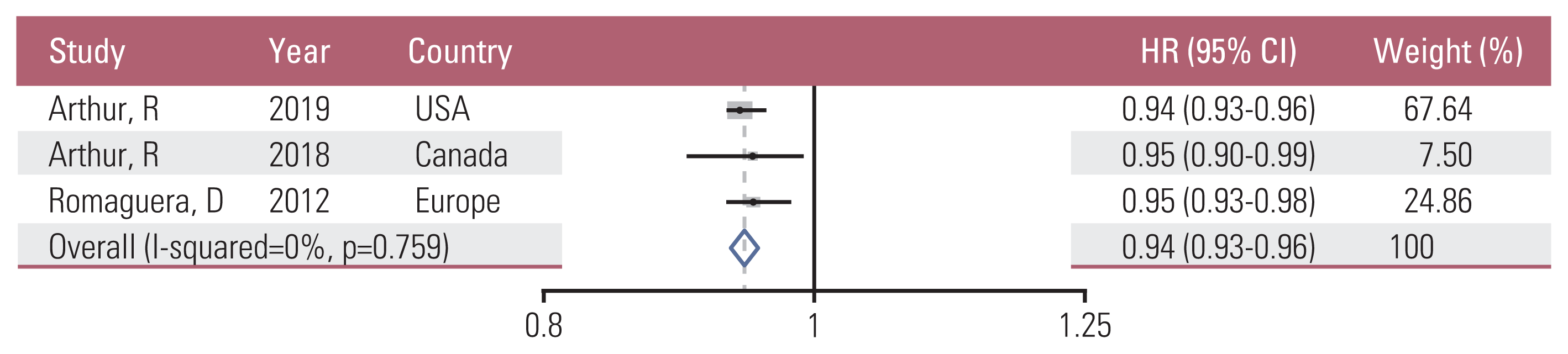
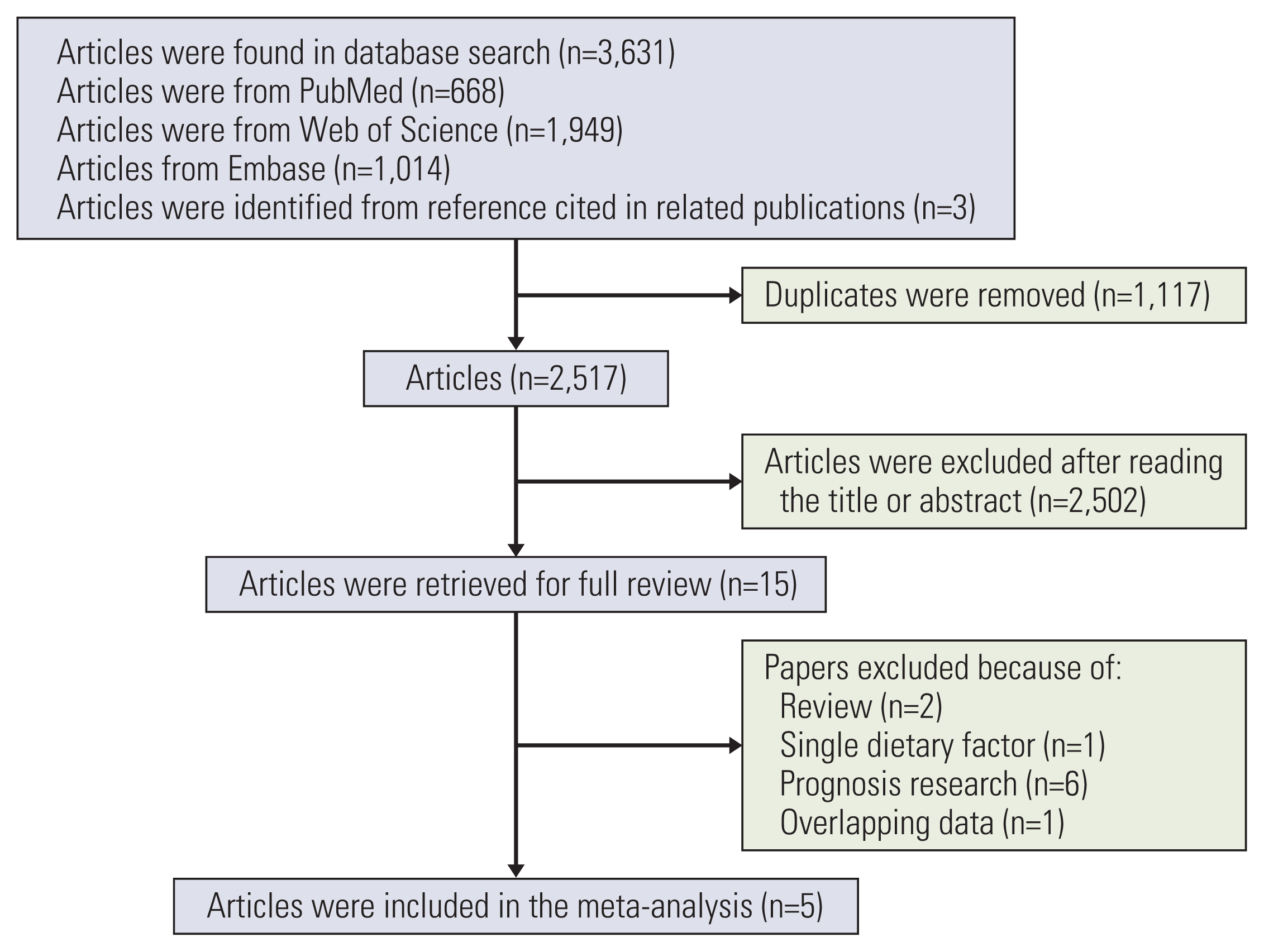
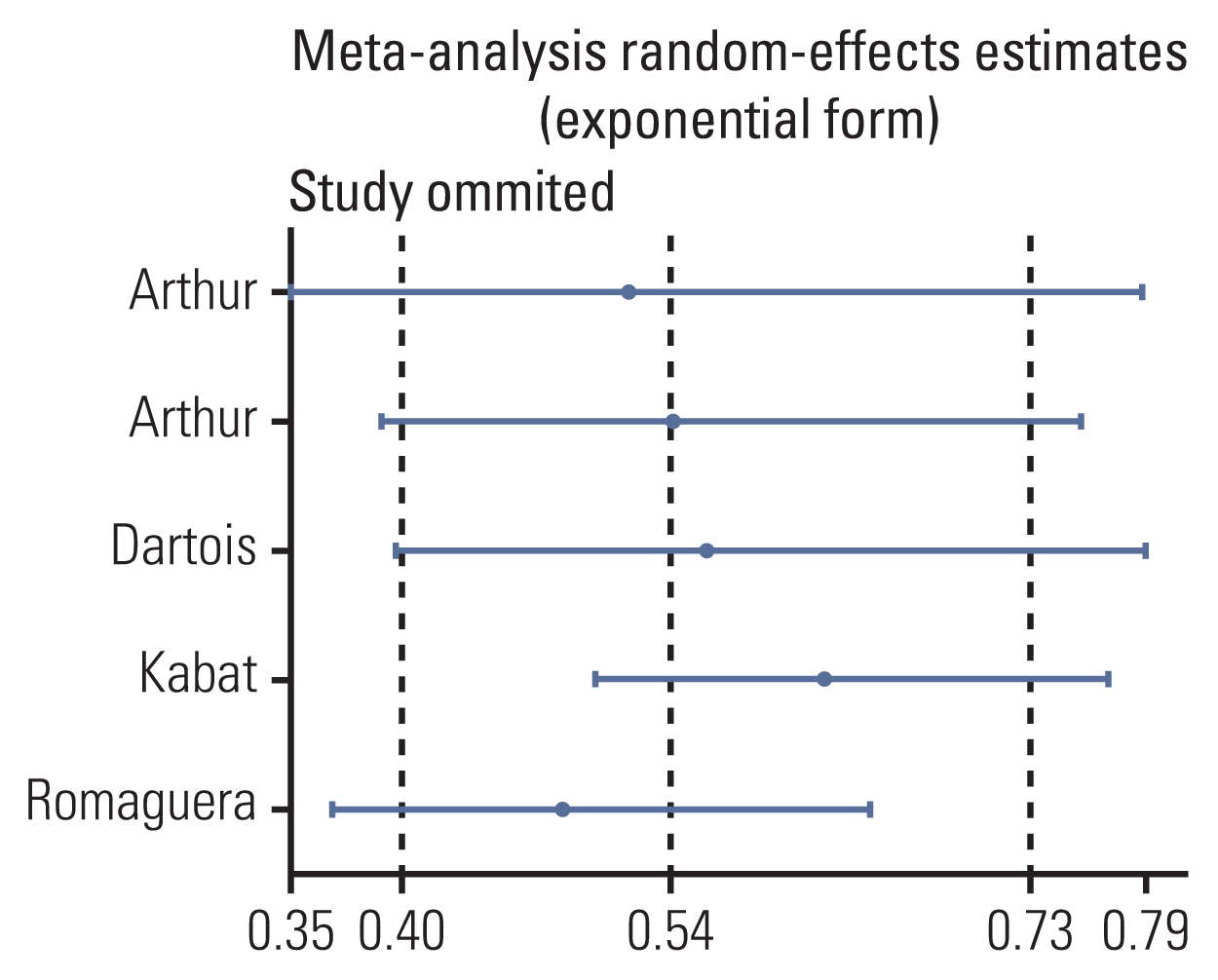
12. Arthur R, Brasky TM, Crane TE, Felix AS, Kaunitz AM, Shadyab AH, et al. Associations of a healthy lifestyle index with the risks of endometrial and ovarian cancer among women in the Women’s Health Initiative Study. Am J Epidemiol. 2019; 188:261–73.

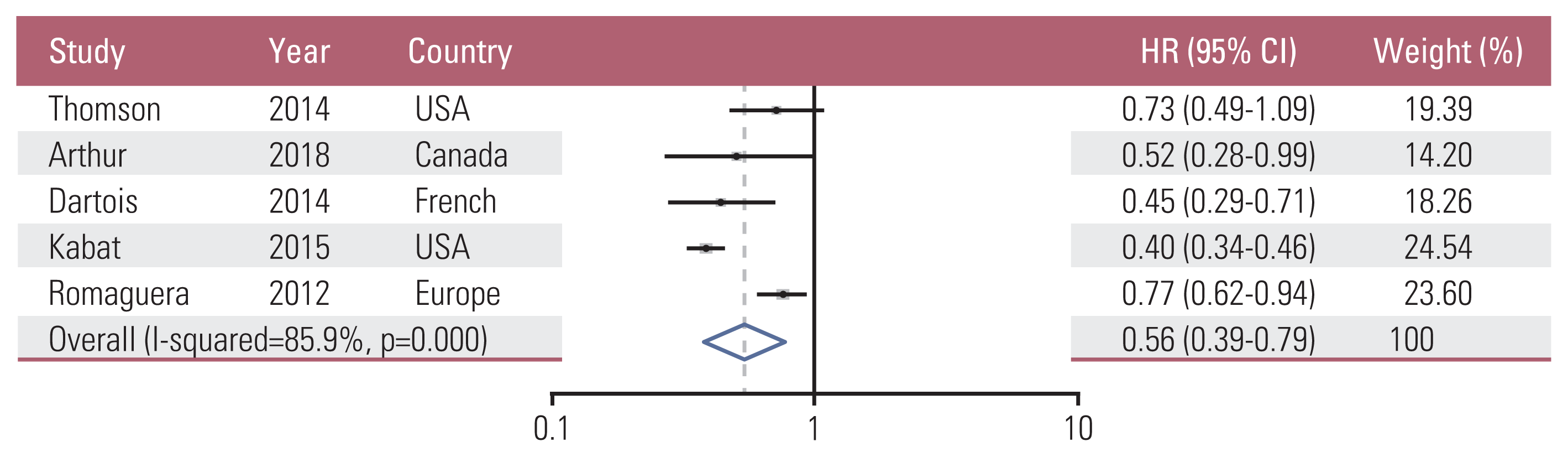
13. Arthur R, Kirsh VA, Kreiger N, Rohan T. A healthy lifestyle index and its association with risk of breast, endometrial, and ovarian cancer among Canadian women. Cancer Causes Control. 2018; 29:485–93.

14. Dartois L, Fagherazzi G, Boutron-Ruault MC, Mesrine S, Clavel-Chapelon F. Association between five lifestyle habits and cancer risk: results from the E3N cohort. Cancer Prev Res (Phila). 2014; 7:516–25.

15. Thomson CA, McCullough ML, Wertheim BC, Chlebowski RT, Martinez ME, Stefanick ML, et al. Nutrition and physical activity cancer prevention guidelines, cancer risk, and mortality in the women’s health initiative. Cancer Prev Res (Phila). 2014; 7:42–53.

16. Kabat GC, Matthews CE, Kamensky V, Hollenbeck AR, Rohan TE. Adherence to cancer prevention guidelines and cancer incidence, cancer mortality, and total mortality: a prospective cohort study. Am J Clin Nutr. 2015; 101:558–69.

17. Romaguera D, Vergnaud AC, Peeters PH, van Gils CH, Chan DS, Ferrari P, et al. Is concordance with World Cancer Research Fund/American Institute for Cancer Research guidelines for cancer prevention related to subsequent risk of cancer? Results from the EPIC study. Am J Clin Nutr. 2012; 96:150–63.

18. Wells GA, Shea B, O’Connell D, Peterson J, Welch V, Losos M, et al. The Newcastle-Ottawa Scale (NOS) for assessing the quality of nonrandomised studies in meta-analyses. Ottawa: University of Ottawa;2013.
19. Gong TT, Wu QJ, Lin B, Ruan SK, Kushima M, Takimoto M. Observational studies on the association between post-diagnostic metformin use and survival in ovarian cancer: a systematic review and meta-analysis. Front Oncol. 2019; 9:458.

20. DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials. 1986; 7:177–88.

21. Greenland S, Longnecker MP. Methods for trend estimation from summarized dose-response data, with applications to meta-analysis. Am J Epidemiol. 1992; 135:1301–9.

22. Chang SC, Lacey JV Jr, Brinton LA, Hartge P, Adams K, Mouw T, et al. Lifetime weight history and endometrial cancer risk by type of menopausal hormone use in the NIH-AARP diet and health study. Cancer Epidemiol Biomarkers Prev. 2007; 16:723–30.

23. Lee SC, Kaunitz AM, Sanchez-Ramos L, Rhatigan RM. The oncogenic potential of endometrial polyps: a systematic review and meta-analysis. Obstet Gynecol. 2010; 116:1197–205.

24. Kushi LH, Doyle C, McCullough M, Rock CL, Demark-Wahnefried W, Bandera EV, et al. American Cancer Society guidelines on nutrition and physical activity for cancer prevention: reducing the risk of cancer with healthy food choices and physical activity. CA Cancer J Clin. 2012; 62:30–67.

25. Kushi LH, Byers T, Doyle C, Bandera EV, McCullough M, McTiernan A, et al. American Cancer Society Guidelines on Nutrition and Physical Activity for cancer prevention: reducing the risk of cancer with healthy food choices and physical activity. CA Cancer J Clin. 2006; 56:254–81.

26. Giovannucci E. Insulin, insulin-like growth factors and colon cancer: a review of the evidence. J Nutr. 2001; 131:3109S–20S.

27. Lee C, Kim SJ, Na JY, Park CS, Lee SY, Kim IH, et al. Alterations in promoter usage and expression levels of insulin-like growth factor-II and H19 genes in cervical and endometrial cancer. Cancer Res Treat. 2003; 35:314–22.

28. Renehan AG, Tyson M, Egger M, Heller RF, Zwahlen M. Body-mass index and incidence of cancer: a systematic review and meta-analysis of prospective observational studies. Lancet. 2008; 371:569–78.

29. Gunter MJ, Hoover DR, Yu H, Wassertheil-Smoller S, Manson JE, Li J, et al. A prospective evaluation of insulin and insulin-like growth factor-I as risk factors for endometrial cancer. Cancer Epidemiol Biomarkers Prev. 2008; 17:921–9.

30. Giovannucci E. Nutrition, insulin, insulin-like growth factors and cancer. Horm Metab Res. 2003; 35:694–704.





 PDF
PDF Citation
Citation Print
Print


 XML Download
XML Download