Introduction
Materials and Methods
1. Tumor cell lines
2. Lentivirus production and stable cell line establishment
3. Cell proliferation assay
4. Western blotting
5. Immunofluorescence assay
6. Flow cytometry apoptosis assay
7. Immunohistochemistry staining
8. Animal models and in vivo tumor formation
9. Statistical analysis
Results
1. IDH1 mutation inhibited TMZ treated glioma cells proliferation
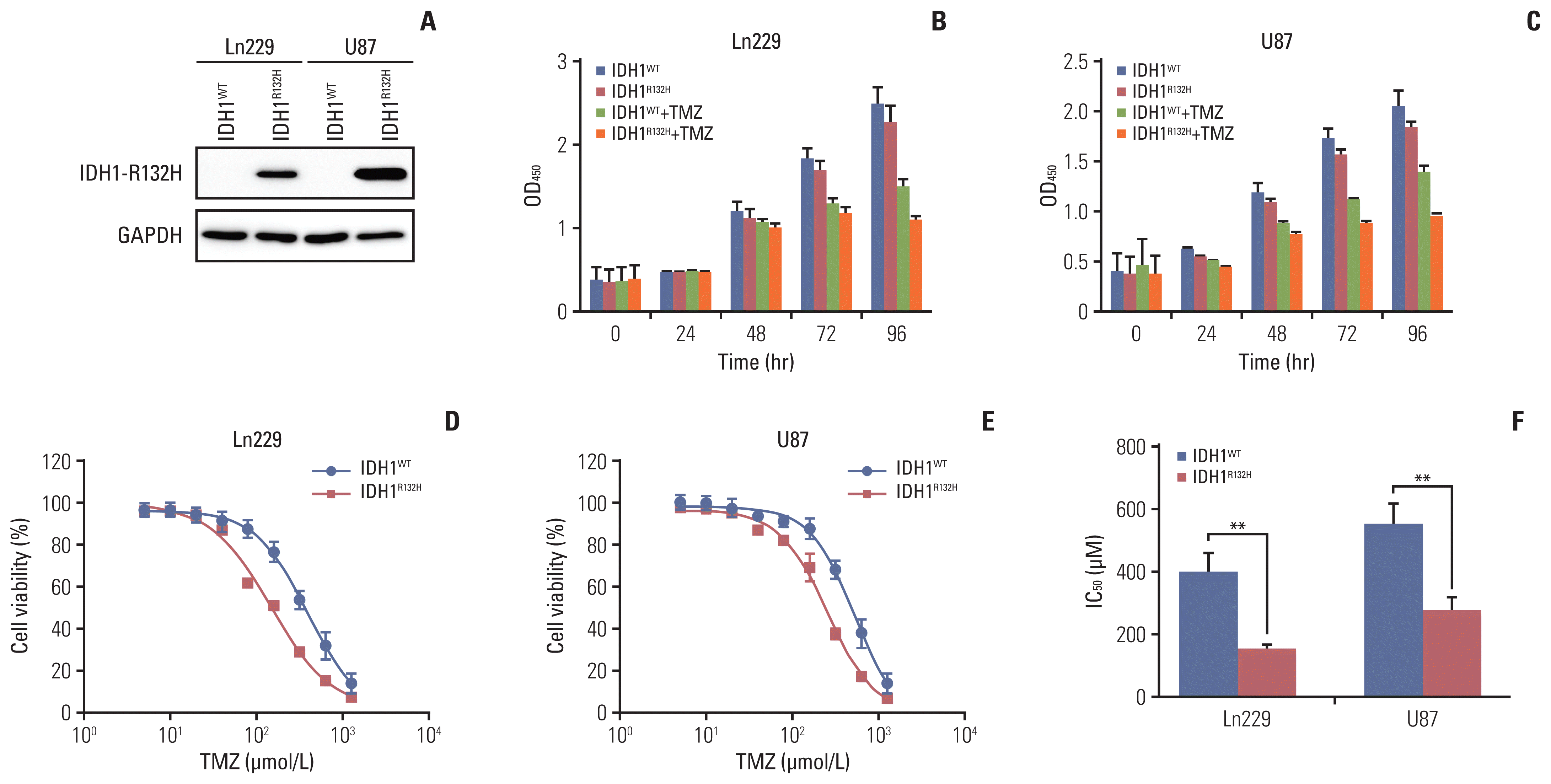 | Fig. 1Isocitrate dehydrogenase 1 (IDH1) mutation inhibits temozolomide (TMZ) treated glioma cells proliferation. (A) IDH1 mutant cells that stably expressing IDH1-R132H mutants in Ln229 and U87 cell lines. IDH1-R132H expression was confirmed by western blot. GAPDH, glyceraldehyde 3-phosphate dehydrogenase. (B, C) IDH1 mutant or IDH1 wild-type cells were treated with or without TMZ for 0 to 96 hours. Cell proliferation was detected by Cell Counting Kit-8. (D, E) TMZ dose-response curves of Ln229 and U87 cells with IDH1 mutation for 96 hours. (F) Statistical analysis of IC50 in Ln229 and U87 glioma cells. **p < 0.01. |
2. TMZ induced more DNA damage in IDH1 mutant glioma
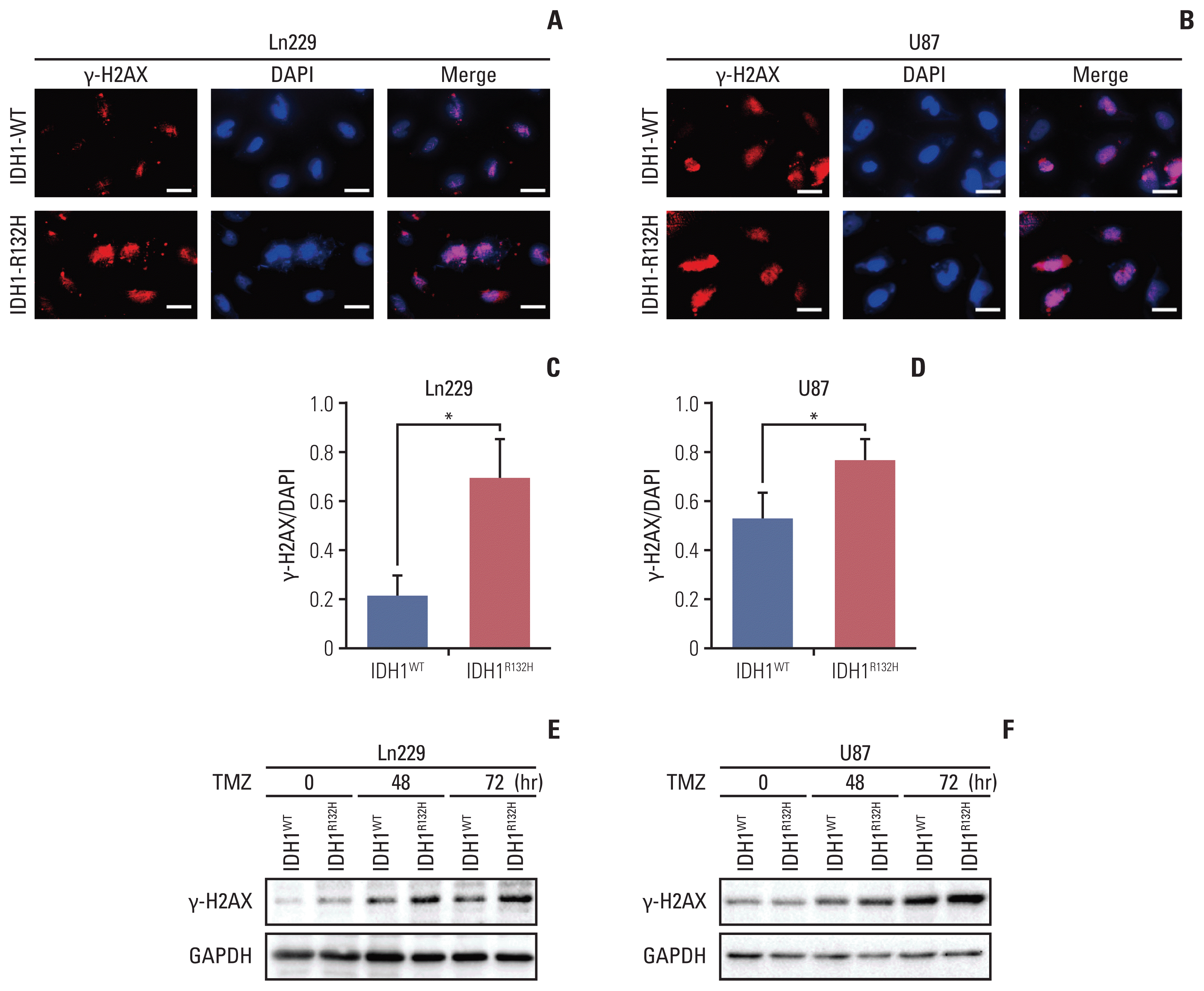 | Fig. 2Temozolomide (TMZ) induced more DNA damage in isocitrate dehydrogenase 1 (IDH1) mutation cells. (A, B) Immunofluorescence staining of γH2AX in glioma cells Ln229 and U87. Scale bars=100 μm. (C, D) Quantification of γH2AX is shown in A and B. *p < 0.05. (E, F) γH2AX expression in glioma cells Ln229 and U87 were detected by western blotting. GAPDH, glyceraldehyde 3-phosphate dehydrogenase. |
3. IDH1 mutation enhanced TMZ induced apoptosis
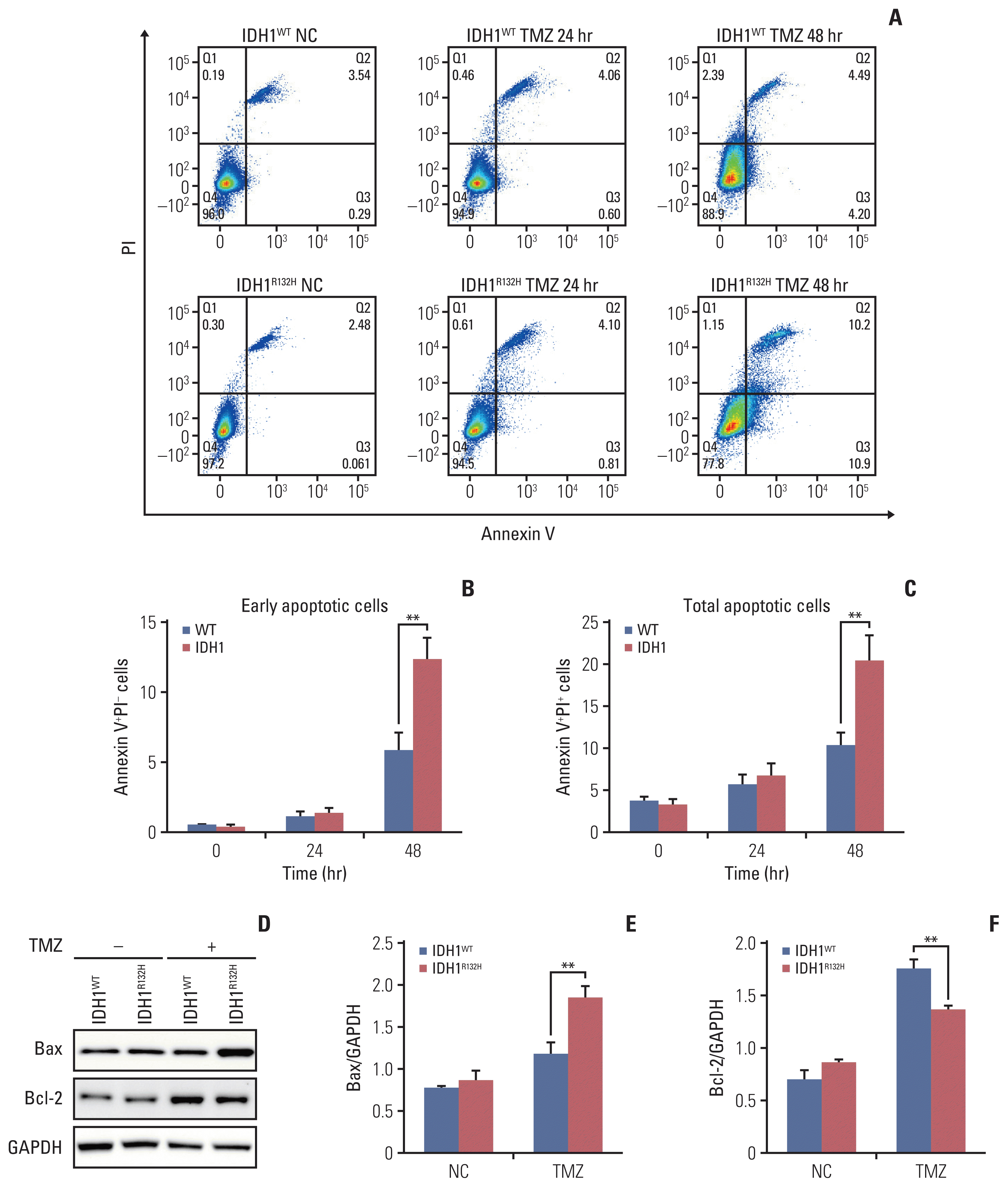 | Fig. 3Isocitrate dehydrogenase 1 (IDH1) mutant enhance temozolomide (TMZ) induced apoptosis. (A) Flow cytometry apoptosis analysis of IDH1 mutant and wild-type cells treated TMZ at different time points. (B) Statistical quantification of annexin V+propidium iodide (PI)− cells. (C) Statistical quantification of annexin V+PI+ cells. (D) Apoptosis-related protein Bax and Bcl-2 were detected by western blotting. GAPDH, glyceraldehyde 3-phosphate dehydrogenase. (E, F) Quantification of relative expression of Bax and Bcl-2. **p < 0.01. |
4. IDH1 mutation inhibited ATM/CHK2 signaling triggered by TMZ
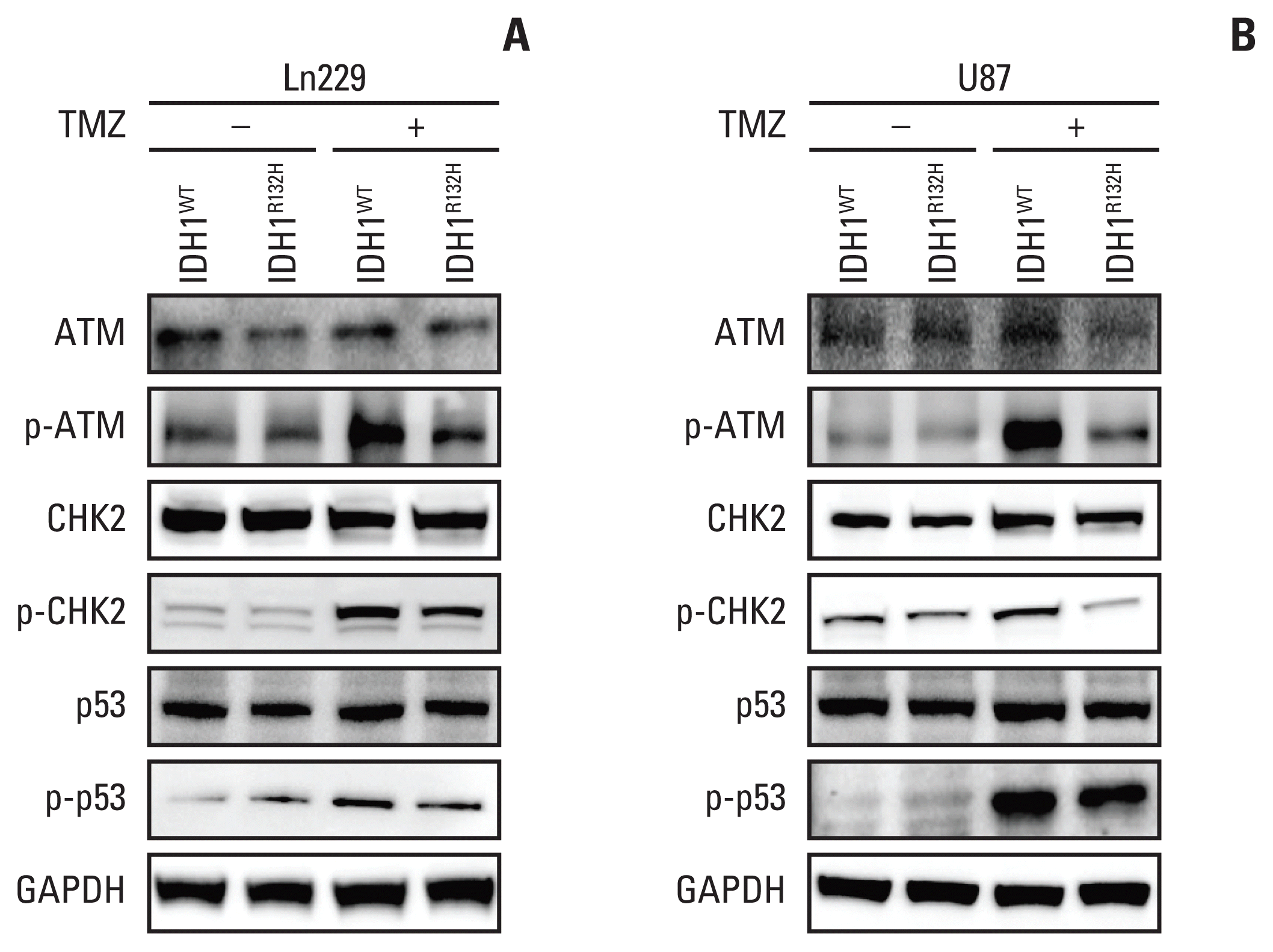 | Fig. 4Isocitrate dehydrogenase 1 (IDH1) mutation inhibits ataxia telangiectasia mutated (ATM)/checkpoint kinase 2 (CHK2) signaling triggered by temozolomide (TMZ). (A, B) The expression of ATM signaling including ATM, CHK2, p53, and their phosphorylated proteins in glioma cells treated with or without TMZ were detected by Western blotting. GAPDH, glyceraldehyde 3-phosphate dehydrogenase. |
5. ATM inhibitor enhanced the antitumor effect of TMZ
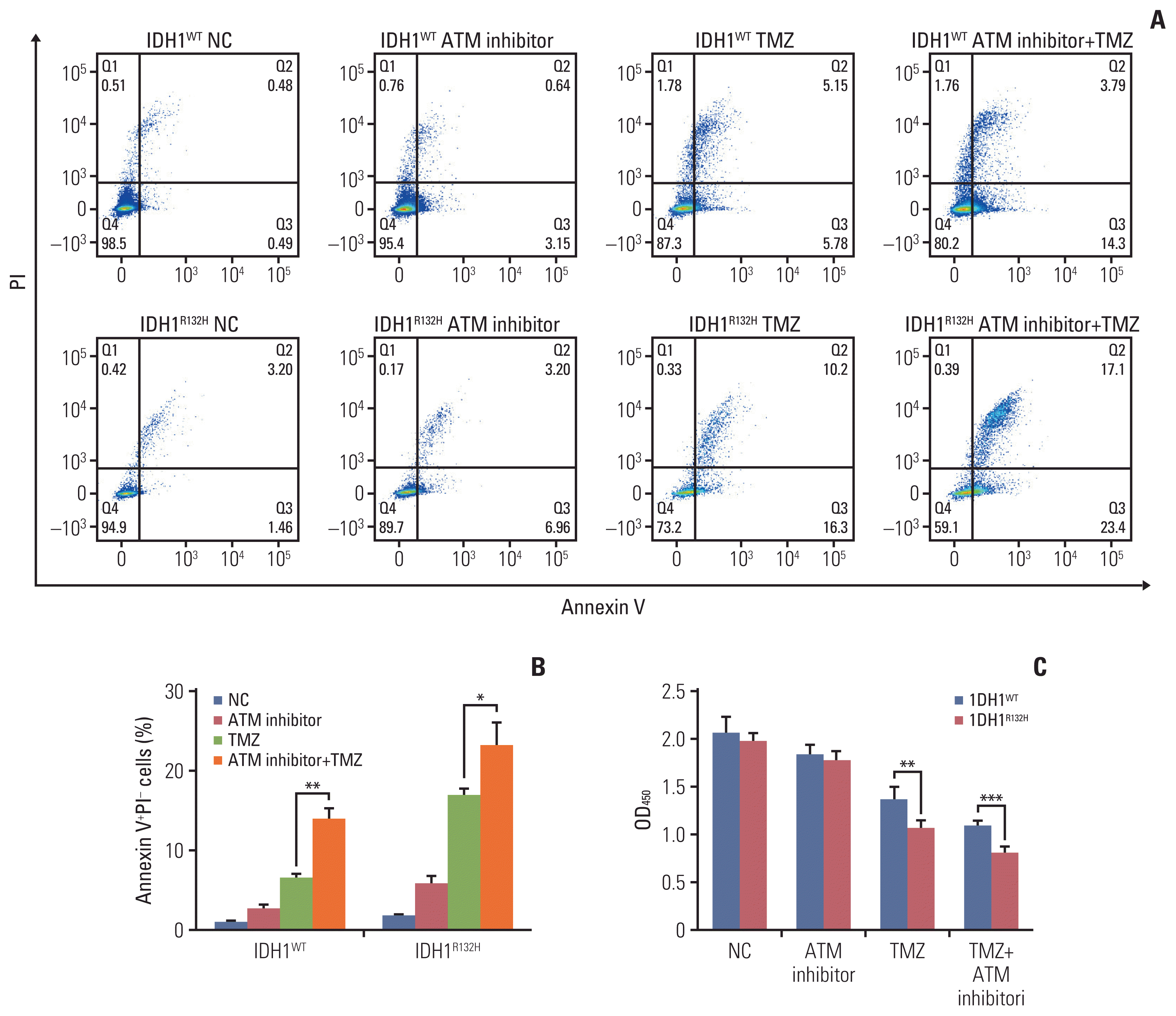 | Fig. 5Ataxia telangiectasia mutated (ATM) inhibitor enhanced the effect of temozolomide (TMZ) on tumor apoptosis. (A) Isocitrate dehydrogenase 1 (IDH1) mutation and wild-type gliomas were divided into four groups treated with negative control, ATM inhibitor, TMZ and ATM inhibitor+TMZ, after 72 hours, cell apoptosis was detected by flow cytometry. (B) Statistical quantification of annexin V+propidium iodide (PI)− cells. (C) IDH1 mutation and wild-type gliomas were treated with negative control, ATM inhibitor, TMZ and ATM inhibitor+TMZ, respectively. Cell proliferation was detected by Cell Counting Kit-8. *p < 0.05, **p < 0.01, ***p < 0.001. |
6. Effect of IDH1 mutation on tumor in vivo
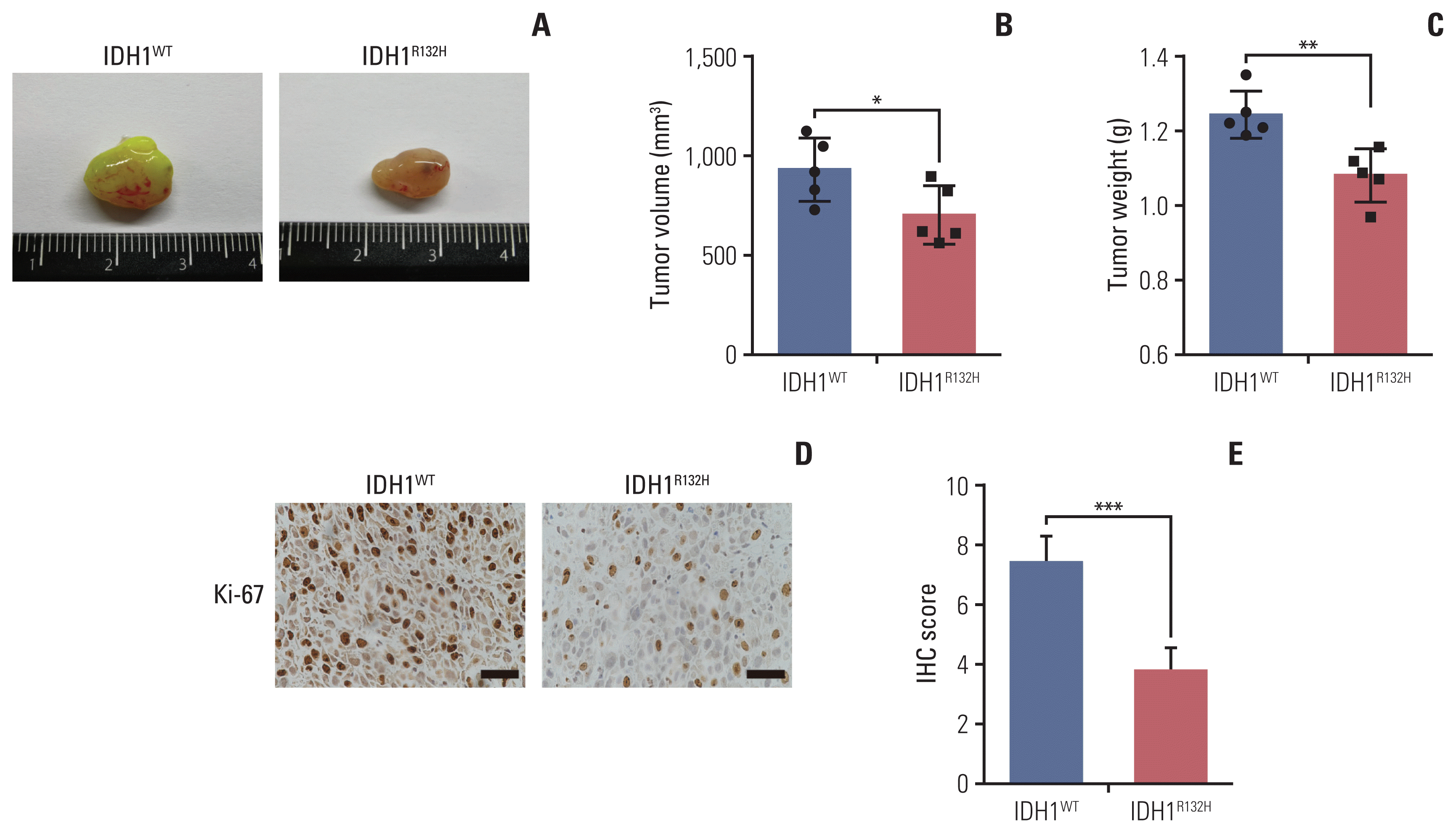 | Fig. 6The effect of isocitrate dehydrogenase 1 (IDH1) mutation on tumor in vivo. (A) The same number of IDH1 mutant and wild-type glioma cells were injected into the subcutaneous of 4–6 weeks nude mice, and the mice were killed and photographed in the fifth week. (B) The tumor size was detected and analyzed statistically. (C) The tumor weight was detected and analyzed statistically. (D) The tumor tissue was paraffin-embedded and stained with immunohistochemistry to detect the expression of Ki-67. Scale bars=25 μm. (E) The results of immunohistochemistry were scored and analyzed statistically. *p < 0.05, **p < 0.01, ***p < 0.001. |
7. The effect of TMZ on IDH1 mutant tumor in vivo
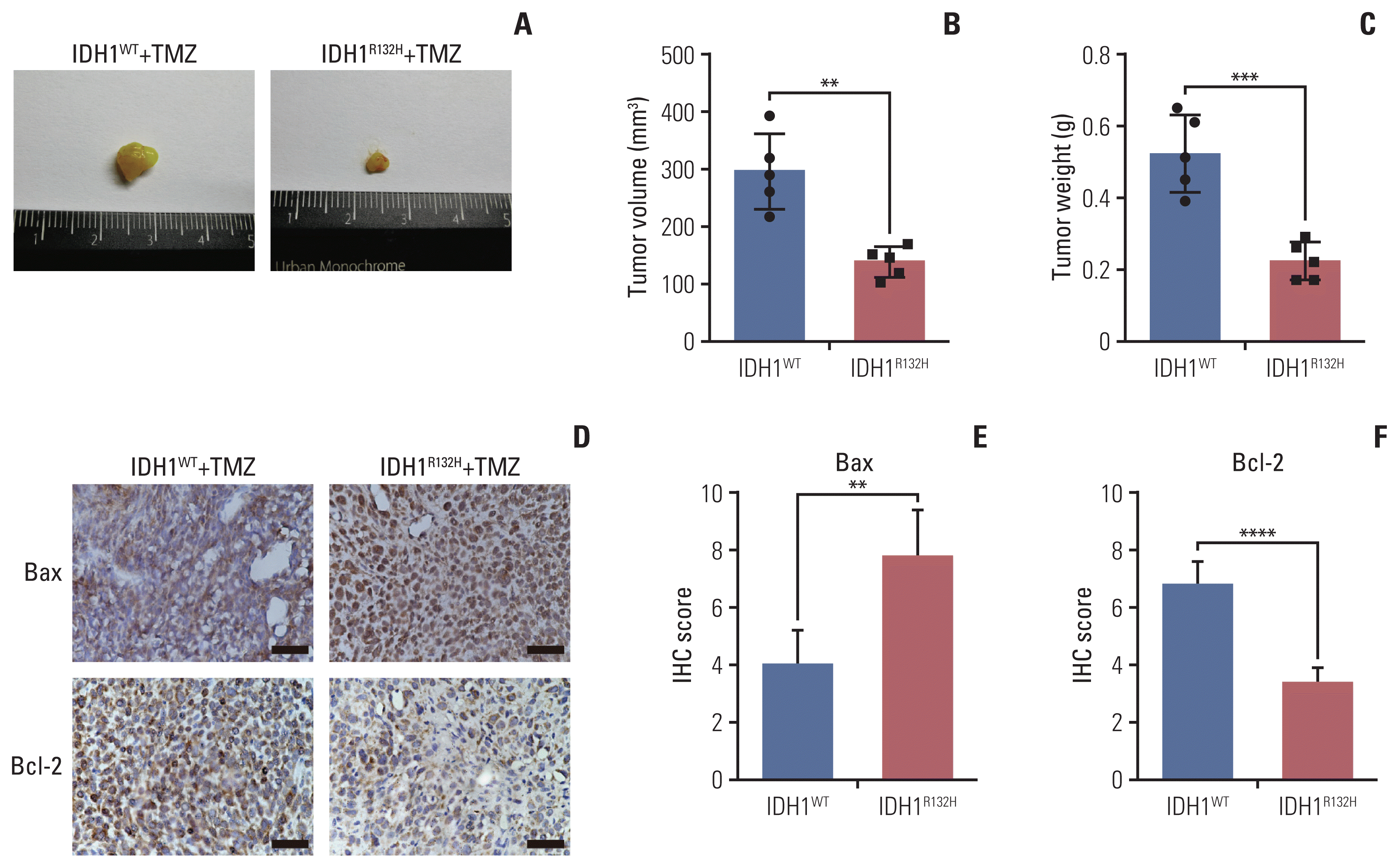 | Fig. 7The effect of temozolomide (TMZ) on isocitrate dehydrogenase 1 (IDH1) mutant glioma in vivo. (A) IDH1 mutant and wild-type glioma cells were subcutaneously injected into 4–6 weeks BALB/c nude mice. After transplanted for 4 weeks 20 mg/kg TMZ was administered intraperitoneally into mice for consecutive 7 days. All nude mice were killed in the sixth week. (B) The tumor volume was monitored and analyzed. (C) The tumor weight was monitored and analyzed. (D) Primary glioma sections from mouse models were stained with Bax and Bcl-2 for immunohistochemistry (IHC) assay. Scale bars=25 μm. (E, F) The results of IHC were scored and analyzed statistically. All results are expressed as the mean±standard deviation; n=5. *p < 0.05, **p < 0.01, ***p < 0.001, ****p < 0.0001. |




 PDF
PDF Citation
Citation Print
Print


 XML Download
XML Download