A 43-year-old woman came to our hospital with loss of consciousness that occurred in the house just before hospital visit. She arrived in the emergency room 45 minutes after symptom occurred. She had no past medical history and was neither smoking nor drinking alcohol. At the time of admission, the vital sign showed a blood pressure of 119/66 mm Hg, a heart rate of 76/min, a respiratory rate of 24/min, and a body temperature of 36.2℃. In neurological examinations, level of consciousness was stuporous, and higher cortical function could not be assessed. Both pupils were dilated 5 mm/7 mm, and no light reflex was seen. The patient had no motor weakness, deep tendon reflex was normoreflexia, no pathologic reflex shown. In the emergency room, brain computed tomography (CT), CT angiography, and CT perfusion were performed. Although there were no remarkable findings on brain CT (
Fig. 1A) and no stenosis or occlusion on brain CT angiography (
Fig. 1E), brain CT perfusion showed mildly decreased cerebral blood flow and cerebral blood volume in left midbrain (
Fig. 1B-
1D). We started to infuse tissue plasminogen activator (tPA) intravenously after 1 hour and 40 minutes from symptom onset. Because there was no occluded vessel on brain CT angiography, we did not consider further treatment like mechanical thrombectomy. In laboratory test, protein C activity was decreased to 31% (normal range, 55% to 123%), cancer antigen-125 (CA-125) was mildly elevated to 39.5 U/mL (normal range, <35). However, there were no more abnormal results in complete blood count, lipid profiles, hemoglobin A1c, thyroid function, liver, and renal function. Other blood tests related to hypercoagulability and vasculopathies including protein S activity, antithrombin III, prothrombin, antiphospholipid antibody, anticardiolipin antibody, lupus anticoagulant, antinuclear antibody, anti-beta2-glycoprotein 1 (GP 1) antibody showed negative. In magnetic resonance imaging including diffusion weighted image (DWI) and angiography, which was performed after 24 hours from tPA infusion, DWI showed hyperintensity lesions at the right side of the cerebellum, at the bilaterally thalamus, midbrain, pons (
Fig. 2A) and MR angiography showed no stenosis or atherosclerotic changes (
Fig. 2B). The patient admitted to intensive care unit and took anti platelet (aspirin 100 mg/day, clopidogrel 75 mg/day), 3-hydroxy-3-methylglutaryl coenzyme A (HMG-CoA) reductase inhibitor (atorvastatin 20 mg) agents for secondary prevention. Her 24 hours holter monitoring and transthoracic echocardiography were all normal. Because CA-125 was elevated in laboratory findings, abdominal pelvis CT was performed to find the presence of pelvic mass. A large sized uterine myoma of 7.5 cm was found (
Fig. 3A). Femoral CT angiography was also performed, there were multiple thrombus was found in both common iliac vein and femoral vein (
Fig. 3B). For deep vein thrombosis, the antiplatelet agent was changed to an anticoagulant agent (dabigatran 300 mg/day). Transcranial Doppler was performed but showed normal results. Neither the bubble test nor the transesophageal echocardiography (TEE) was could not be performed because valsalva maneuver could not be performed due to stuporous mentality. Protein C activity was rechecked 3 months later and the result was restored to normal as 64%.
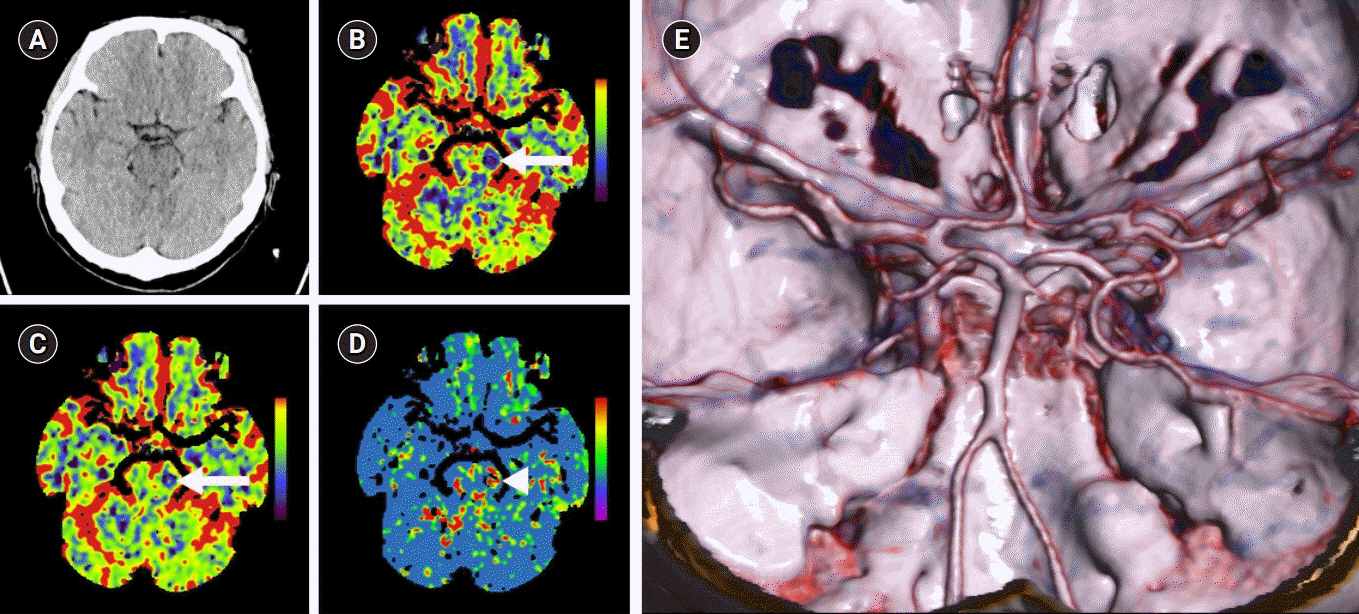 | Fig. 1.Brain computed tomography (CT) and CT angiography were performed immediately after arrival at the emergency room. (A) There was no remarkable finding on brain CT. (B) Cerebral blood flow and (C) cerebral blood volume map shows decreased perfusion of the left midbrain (arrows). (D) Mean transit time map shows a prolongation within the same region (arrowhead), indicative of core infarct in the left midbrain. (E) There were no stenosis or occlusion on basilar artery and the other vessels. 
|
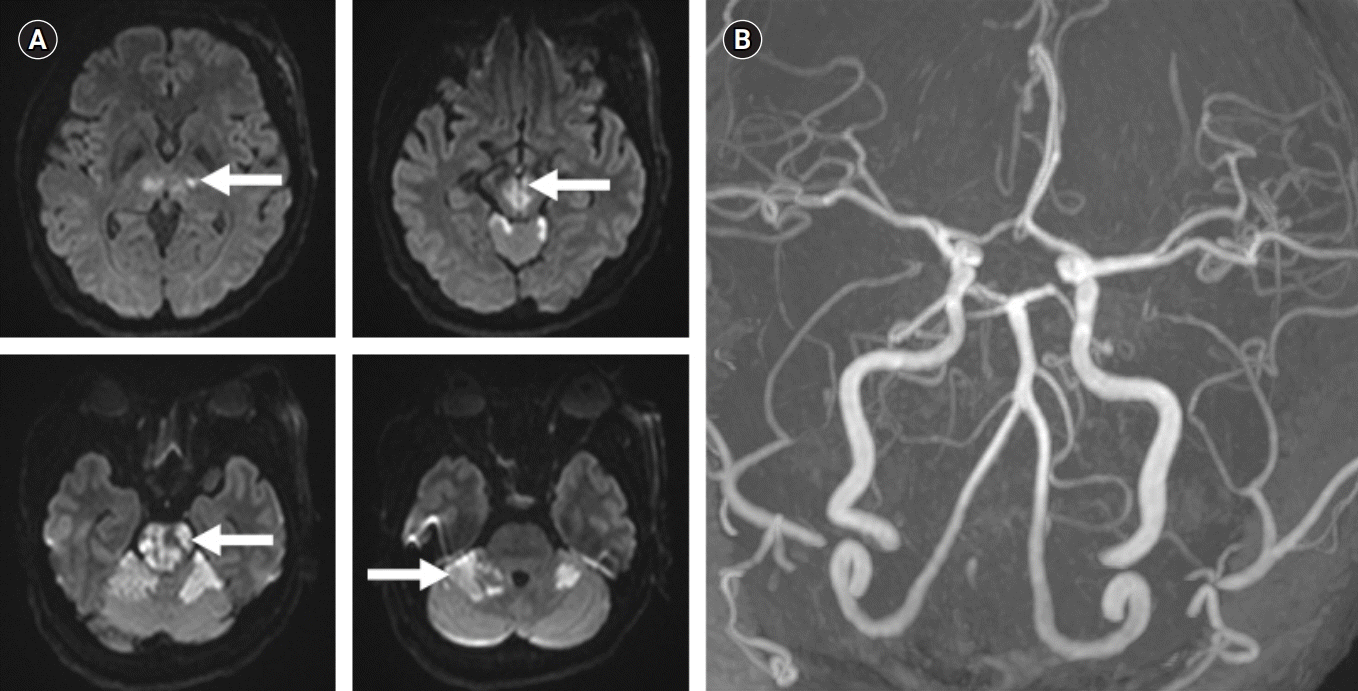 | Fig. 2.(A) Diffusion-weighted imaging, performed after 1 day from symptom onset, showed acute ischemic lesion in the bilateral thalamus, midbrain, pons and right cerebellum (arrows). (B) Magnetic resonance angiography which was performed after 1 day from symptom onset, showed no stenosis or occlusion. 
|
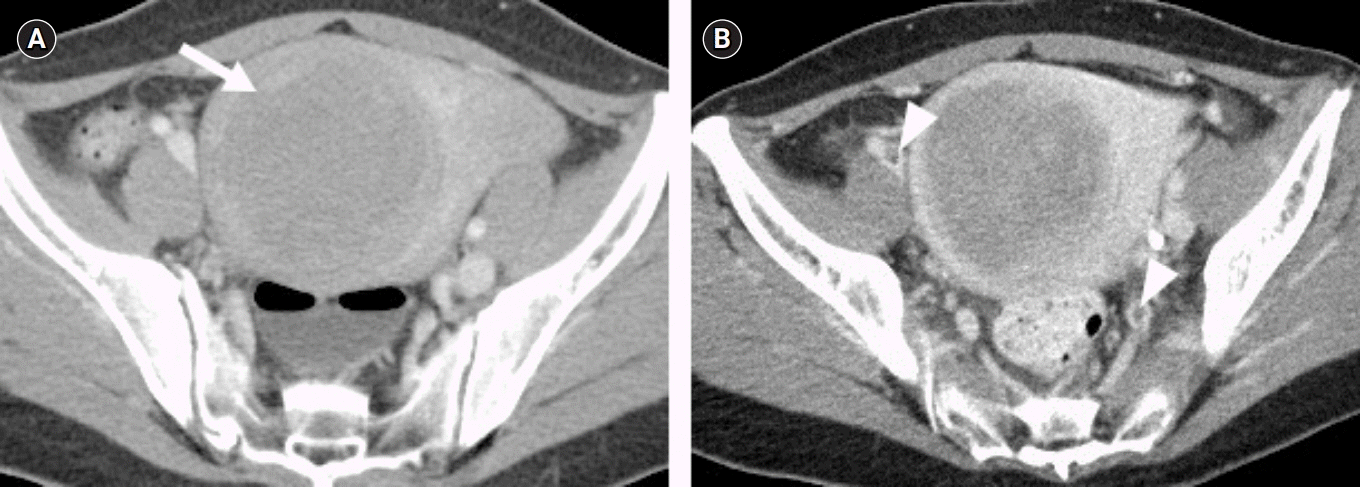 | Fig. 3.(A) There was a 7.5 cm uterine myoma with several small myomas in pelvic cavity (arrow). (B) Femoral computed tomographic angiography reveals contrast filling defects in the right external iliac vein, left internal iliac vein (arrowheads). 
|






 PDF
PDF Citation
Citation Print
Print




 XML Download
XML Download