Abstract
Background
The neurological manifestations of Sjögren’s syndrome (SjS) are nonspecific and may precede the onset of sicca symptoms. Hence, the diagnosis of SjS is often delayed. Recurrent aseptic meningitis is an uncommon neurological manifestation of primary SjS; only few cases have been reported in the medical literature.
Case Report
A 54-year-old woman was admitted for recurrent aseptic meningitis. The patient had a history of two episodes of aseptic meningitis, which had occurred 12 and 7 years before this presentation. The patient had overt sicca symptoms for 5 years. SjS was diagnosed based on the results of serum autoantibody tests, Schirmer’s test, and salivary scintigraphy. We concluded that recurrent aseptic meningitis occurred as an initial presentation of primary SjS.
Go to : 
Sjögren’s syndrome (SjS) is a chronic autoimmune disease that may be primary or secondary to other connective tissue disorders, particularly rheumatoid arthritis, systemic lupus erythematosus, and systemic sclerosis. SjS is clinically characterized by sicca symptoms, which develop due to a mononuclear infiltration and secondary chronic dysfunction of the lacrimal and salivary glands. Systemic, extra-glandular manifestations are found in many patients with primary SjS and may occur in almost any organ, including the nervous system. The central nervous system (CNS) involvement includes diffuse abnormalities (cognitive impairment, psychiatric changes, and aseptic meningitis), and focal or multifocal involvement of the brain and spinal cord (seizures, cerebellar syndrome, myelopathy, and optic neuropathy) [1-4]. However, recurrent aseptic meningitis is an uncommon neurological manifestation of primary SjS; only few cases have been published in the medical literature, and none have been reported in Korean literature [4,5]. Herein, we report the case of a 54-year-old woman with recurrent aseptic meningitis associated with primary SjS.
Go to : 
A 54-year-old woman was admitted owing to headache, nausea, and fever following mild flu-like symptoms for 7 days. On admission, her body temperature was 38.2°C, blood pressure was 110/60 mm Hg, and pulse rate was 80/min. The physical examination revealed mild nuchal rigidity. The remainder of the examination was normal. The patient had a history of two episodes of aseptic meningitis, which had occurred 12 and 7 years before this presentation (Table 1). For the previous two episodes of aseptic meningitis, the patient was treated only with supportive care (intravenous hydration and nonsteroidal antiinflammatory drugs for pain control) and was discharged within 2 weeks. Her medical history was otherwise unremarkable. The laboratory blood tests revealed a mild normochromic anemia (hemoglobin level, 10.2 g/dL), and an elevated erythrocyte sedimentation rate (120 mm/hr). The analysis of the cerebrospinal fluid (CSF), obtained by lumbar puncture, revealed a crystal-clear appearance, mildly elevated opening pressure of 170 mm H2O, moderate pleocytosis (170/mm3, 51% lymphocytes), and normal protein (49.43 mg/dL) and glucose levels (52 mg/dL).
Laboratory and radiologic findings in the three episodes of aseptic meningitis
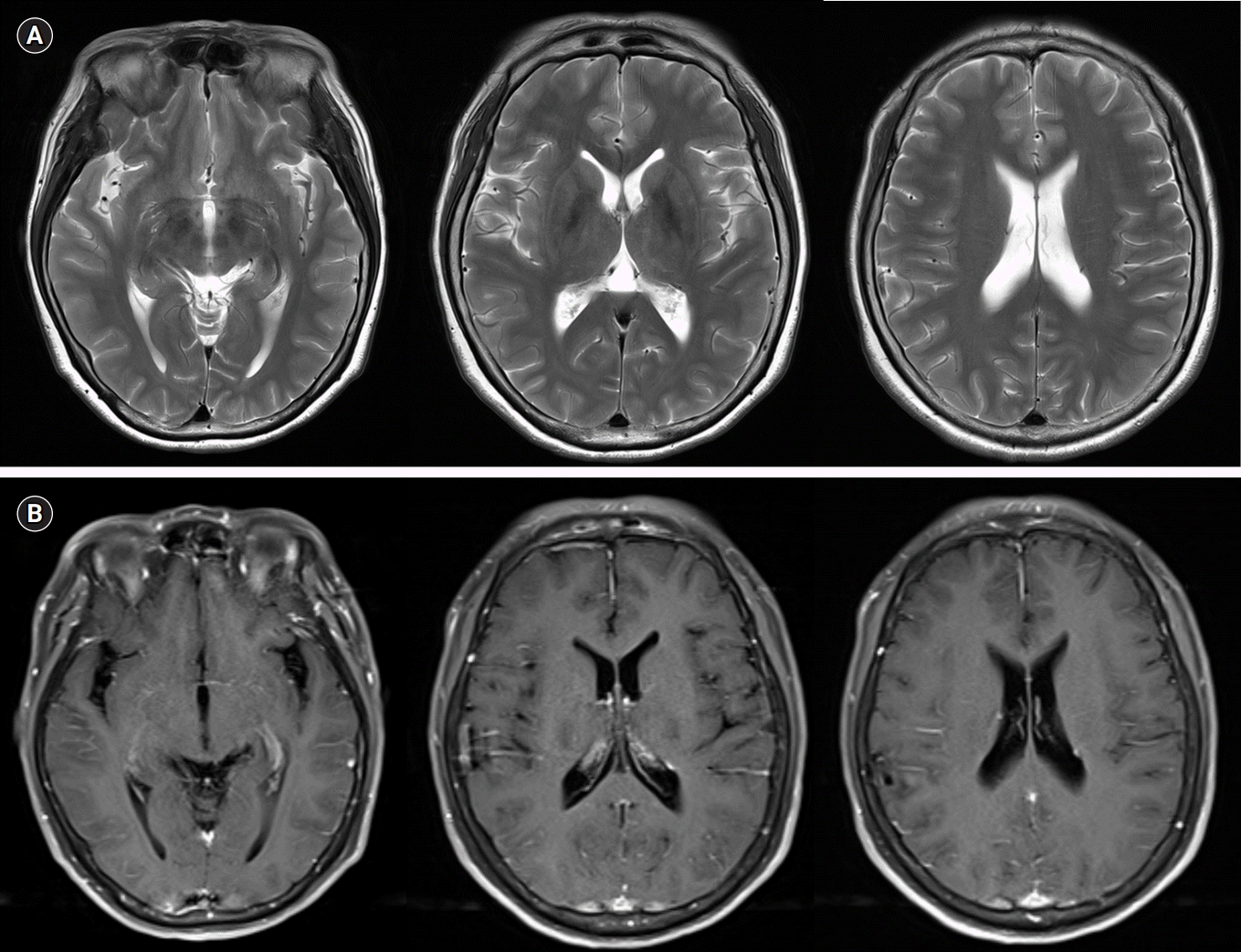
The patient was initially suspected to have recurrent benign lymphocytic meningitis associated with herpes simplex virus (HSV) type 2 and was started on a treatment with intravenous acyclovir at a dose of 30 mg/kg daily. The tests for an infectious etiology, polymerase chain reaction for HSV type 1 and 2 and varicella-zoster virus, were negative, and cultures for bacteria, mycobacterium, and fungi were also negative. Cerebral magnetic resonance imaging did not reveal any abnormalities (Fig. 1). Five days after admission, the patient recovered to normal condition without fever, headache, or nuchal rigidity. Acyclovir was stopped after 7 days of administration due to no evidence of HSV infection. The patient was discharged to her home 8 days after admission in a stable condition.
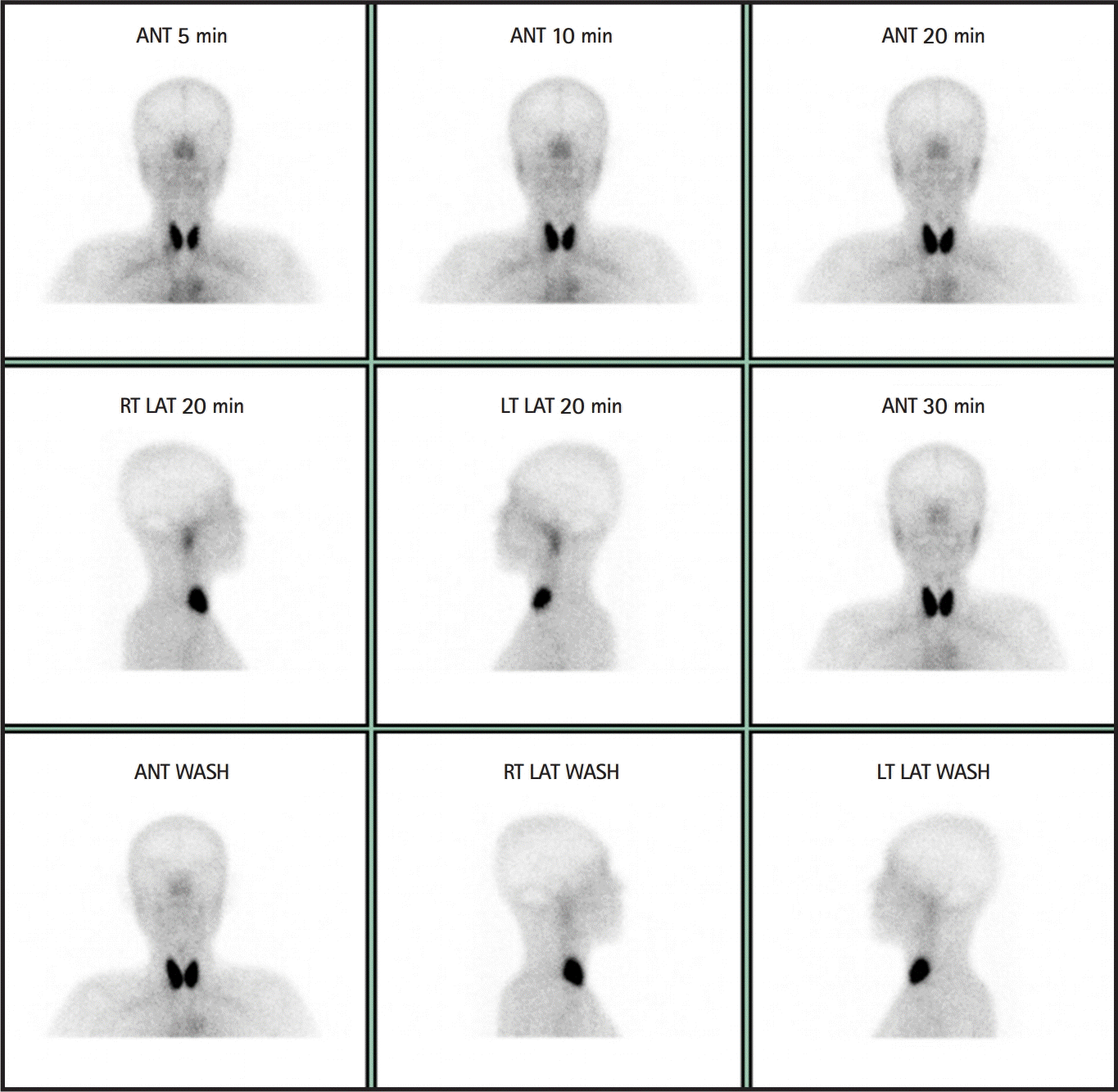
For evaluation of the relapsing meningitis, an investigation for systemic autoimmune diseases was conducted. Anti-Ro/SS-A, anti-La/SS-B, as well as anti-nuclear antibodies resulted positive in the serum. Rheumatoid factor, anti-Smith, and anti-double-stranded DNA (dsDNA) antibody tests were negative. Through a more detailed medical history taking, it was determined that the patient has been using artificial tears for ocular dryness for 5 years. She also complained that it was difficult to swallow without water when eating dry food in the last 5 years. She had chronic fatigue, but denied Raynaud’s phenomenon, arthralgia, myalgia, and photosensitivity. At this point, primary SjS was suspected, and more specific investigations were performed for diagnostic confirmation. Schirmer’s test showed a decreased tear secretion rate in the left eye (5 mm of filter paper moistened for 5 minutes). In addition, scintigraphy of the salivary glands showed nearly non-functioning parotid glands and submandibular gland (Fig. 2). Therefore, the patient was diagnosed with SjS and recurrent aseptic meningitis. We consulted a rheumatologist, and oral pilocarpine medication was started for the sicca symptoms. The patient is followed up in the rheumatology outpatient clinic.
Go to : 
This case report describes a patient with recurrent aseptic meningitis, which preceded the diagnosis of SjS. Our patient was diagnosed with SjS according to the 2016 criteria proposed by the American College of Rheumatology/European League Against Rheumatism (ACR-EULAR), which is the most recently used standard for classification in clinical and scientific research [6]. The patient had ocular and oral symptoms for 5 years, positive anti-Ro/SS-A antibodies, and abnormal Schirmer’ test. In addition, the diagnosis of SjS was excluded in the item of the 2016 ACR-EULAR criteria; however, the abnormal scintigraphy supported this diagnosis.
Recurrent meningitis is rarely seen; however, when it occurs, a detailed investigation for determining the pathogenesis underlying the recurrent episodes must be pursued. Causes of recurrent meningitis fall into five main categories: infections, malignant neoplasms, benign tumors, drugs, and autoimmune diseases [7]. Recurrent aseptic meningoencephalitis as a neurological manifestation of primary SjS was first reported by Alexander and Alexander [4] in 1983. The exact pathogenesis of aseptic meningitis in SjS is still not fully understood. Hypersensitivity to some agents, such as viruses, drugs, or others, and the presence of oligoclonal bands, elevated immunoglobulin G levels, and CSF antibodies that cross-react with the meningeal cells is hypothesized to cause CNS lymphocyte infiltration and autoantibody production [5].
In our case, the diagnosis of SjS was delayed because the neurological manifestation preceded the overt sicca symptoms, which is the reason that investigations for SjS were not performed during the two prior episodes of meningitis. Because CNS involvement frequently predates the onset of sicca symptoms in SjS and often transpires as recurrent, multifocal episodes separated by long disease-free intervals, the diagnosis can often be very challenging [1]. According to previous studies, neurological manifestations may precede the typical sicca symptoms in 25% to 92% of the cases [8,9]. In a study of 82 patients with primary SjS with neurologic manifestations, 66 (81%) patients were diagnosed after the onset of neurologic symptoms, with a mean delay of 4.6 years [1]. To reflect these points, the most recent 2016 ACR-EULAR classification for primary SjS excludes the subjective ocular and oral symptoms [6].
The early and accurate diagnosis of SjS can help prevent or ensure adequate treatment of the many complications associated with the disease. It is well known that there is an increased risk of lymphoproliferative disease development as a severe complication of primary SjS. The risk of B-cell non-Hodgkin lymphoma occurrence in the setting of SjS has been previously estimated to be 7- to 19-fold higher than that in the general population [10].
At present, there is no treatment modality that can cure primary SjS. Hence, symptomatic treatment for the sicca symptoms is commonly used. However, in case of significant systemic involvement, including neurological manifestation, immunomodulatory or immunosuppressive therapy can be applied, despite the limited evidence in small, uncontrolled case series [11,12].
Our case report has some limitations. It is our presumptive diagnosis that primary SjS was the cause of recurrent aseptic meningitis. Particularly, two episodes of meningitis before the onset of sicca symptoms cannot be attributed to SjS. However, other known causes for recurrent meningitis, such as other autoimmune diseases, drugs, benign tumors, and malignancies, could be excluded when considering our patient’s clinical history, laboratory findings, and clinical course.
In conclusion, this case highlights that SjS should be considered in the differential diagnosis of recurrent aseptic meningitis. In addition, the diagnostic approach should include both a detailed medical history taking and serological examinations, including immunological screening.
Go to : 
Notes
Author contributions
Conceptualization: SJL and DHL. Data acquisition & Formal analysis: SJL and DHL, Visualization & Writing–origial draft: SJL and DHL. Writing–review editing: SJL and DHL.
Go to : 
REFERENCES
1. Delalande S, de Seze J, Fauchais AL, Hachulla E, Stojkovic T, Ferriby D, et al. Neurologic manifestations in primary Sjögren syndrome: a study of 82 patients. Medicine (Baltimore). 2004; 83:280–91.
2. Tobón GJ, Pers JO, Devauchelle-Pensec V, Youinou P. Neurological disorders in primary Sjögren's syndrome. Autoimmune Dis. 2012; 2012:645967.

3. Rossi R, Valeria Saddi M. Subacute aseptic meningitis as neurological manifestation of primary Sjögren's syndrome. Clin Neurol Neurosurg. 2006; 108:688–91.

4. Alexander EL, Alexander GE. Aseptic meningoencephalitis in primary Sjögren's syndrome. Neurology. 1983; 33:593–8.
5. Ishida K, Uchihara T, Mizusawa H. Recurrent aseptic meningitis: a new CSF complication of Sjögren's syndrome. J Neurol. 2007; 254:806–7.
6. Shiboski CH, Shiboski SC, Seror R, Criswell LA, Labetoulle M, Lietman TM, et al. 2016 American College of Rheumatology/European League Against Rheumatism classification criteria for primary Sjögren's syndrome: a consensus and data-driven methodology involving three international patient cohorts. Ann Rheum Dis. 2017; 76:9–16.
8. Mori K, Iijima M, Koike H, Hattori N, Tanaka F, Watanabe H, et al. The wide spectrum of clinical manifestations in Sjögren's syndrome-associated neuropathy. Brain. 2005; 128(Pt 11):2518–34.

9. Gono T, Kawaguchi Y, Katsumata Y, Takagi K, Tochimoto A, Baba S, et al. Clinical manifestations of neurological involvement in primary Sjögren's syndrome. Clin Rheumatol. 2011; 30:485–90.

10. Fragkioudaki S, Mavragani CP, Moutsopoulos HM. Predicting the risk for lymphoma development in Sjögren syndrome: an easy tool for clinical use. Medicine (Baltimore). 2016; 95:e3766.
11. Saraux A, Pers JO, Devauchelle-Pensec V. Treatment of primary Sjögren syndrome. Nat Rev Rheumatol. 2016; 12:456–71.

12. de Seze J, Delalande S, Fauchais AL, Hachulla E, Stojkovic T, Ferriby D, et al. Myelopathies secondary to Sjögren's syndrome: treatment with monthly intravenous cyclophosphamide associated with corticosteroids. J Rheumatol. 2006; 33:709–11.
Go to : 




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download