Abstract
Background
Cerebral air embolism is uncommon but potentially causes catastrophic events such as cardiac damage or even death. However, due to a low overall incidence, it may go undiagnosed.
Case Report
A 56-year-old man with a medical history of right upper lobectomy due to lung cancer showed changes in mental status after the Valsalva maneuver, followed by status epilepticus during admission. Brain and chest computed tomography showed cerebral air embolism and accidental pneumothorax in the right major fissure. After antiepileptic drug infusion and oxygen therapy, he recovered completely.
Cerebral air embolism is a rare but potentially severe complication of iatrogenic procedures or destructive lung disease, possibly resulting in neurological disorders such as encephalopathy, stroke, or seizure. Due to a low overall incidence, cerebral air embolism may go undiagnosed. We report the case of a patient with cerebral air embolism presenting with status epilepticus after the Valsalva maneuver during a pulmonary function test.
A 56-year-old man with a medical history of right upper lobectomy due to lung cancer presented to the emergency room with changes in mental status after the Valsalva maneuver during a pulmonary function test. He had undergone lobectomy 10 days before the event. The oxygen saturation was 99% by full face mask, and the patient underwent endotracheal intubation because of comatose mental state.
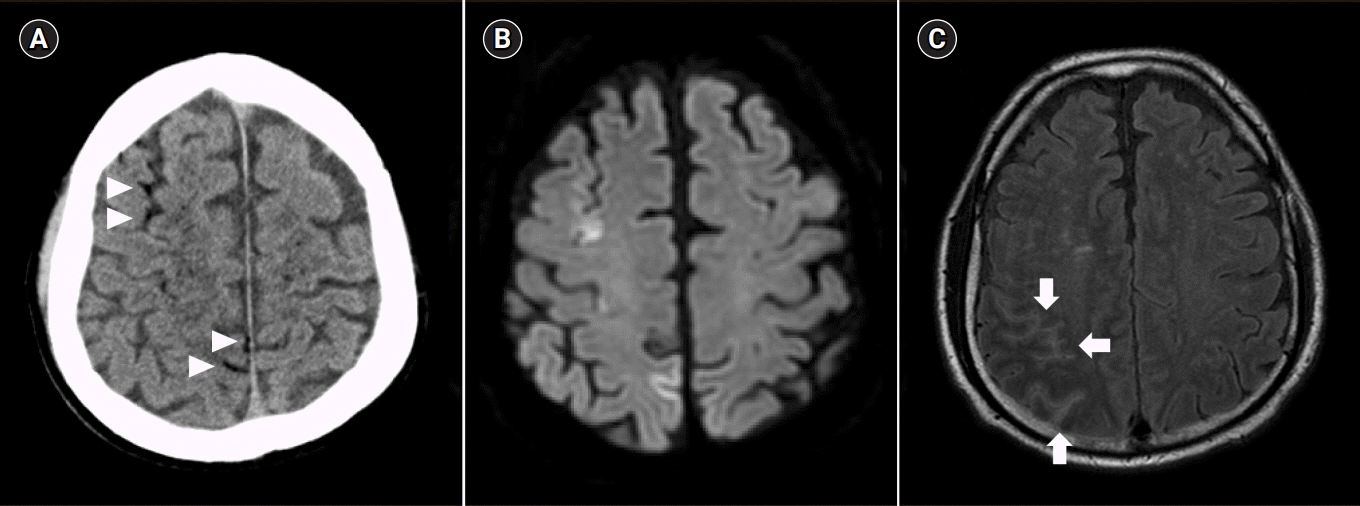
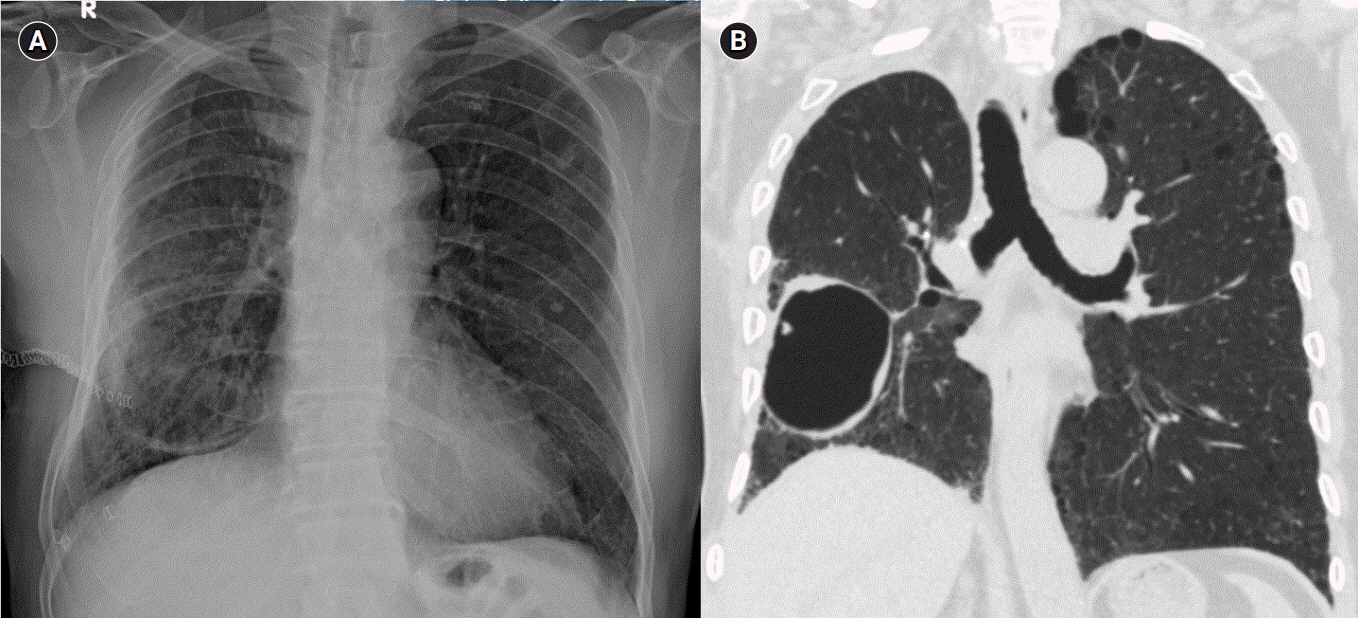
Brain computed tomography (CT) after 30 minutes from the symptom onset revealed scattered air bubbles in the bilateral cerebral subarachnoid space and deep cerebral white matter, suggesting air embolism (Fig. 1A). In addition, chest CT revealed localized pneumothorax in the right major fissure (Fig. 2).
With the intravenous administration of fluid and applied O2 therapy for 8 hours, the patient was able to make eye contact. However, about 11 hours after the event, three attacks of generalized tonic-clonic seizures with right gaze deviation occurred for 40 minutes. The patient did not regain consciousness between the seizures. Suspecting status epilepticus with acute kidney injury, antiepileptic drug with levetiracetam 1,500 mg intravenous was loaded, followed by postictal confusion over 8 hours. Within the 8 hours, he showed tactile and visual extinction, left-sided weakness, and bilaterally positive Babinski signs.
Five days after the seizure, brain magnetic resonance imaging revealed newly appearing diffuse leptomeningeal enhancement of the right frontal, bilateral parietooccipital, and bilateral cerebellum with diffuse enhancement in the perivascular space of bilateral deep white matter (Fig. 1B, 1C). Magnetic resonance angiography revealed mild atherosclerotic changes in the bilateral proximal and cavernous internal carotid arteries and focal stenosis at left M1. Furthermore, a transesophageal echocardiogram revealed patent foramen ovale (PFO) Grade I. Five days after supportive therapies such as mechanical ventilation and oxygen therapy, the patient was discharged from the hospital without any neurological deficits. After discharge, he took levetiracetam 500 mg twice a day daily.
Cerebral air embolism is a rare but life-threatening event, which may cause sudden-onset respiratory distress or neurological events such as seizure and comatose mental status [1,2]. It often occurs with lung trauma or during vascular interventions or surgical procedures such as central venous catheter removal, cardiac surgeries, and endoscopic operations [3,4].
Diagnosis of cerebral air embolism should be based on the patient’s medical history and clinical suspicion. Brain CT may be a valuable tool if the air is visible within the cerebral parenchyma or subarachnoid space early in the disease course. However, there is no established imaging technique for the definite diagnosis of cerebral air embolism [5].
In our case, status epilepticus was caused by acute cerebral damage after cerebral air embolism. Anticonvulsant medication may be required for the control of seizures. Otherwise, prophylactic use of lidocaine not only controls seizures but also reduces infarct size and prevents cardiac arrhythmias associated with air embolism [6].
There are two mechanisms behind cerebral air embolism. One is the retrograde venous cerebral embolism via the venous system [1]. In our case, during the Valsalva maneuver, the increased intrathoracic pressure and tension pneumothorax may have provided a pressure gradient for the air to enter the pulmonary veins, resulting in air embolus. The other is the paradoxical embolism in the presence of right-to-left shunting such as PFO and pulmonary arteriovenous fistula [5,7-10]. The conditions that elevate the pulmonary artery pressure enable the air bubbles to move from right to left. The presence of intracardiac shunt (PFO G1) in this patient may explain the cause of air embolism.
The initial treatment consists of immediate volume expansion, administration of 100% oxygen by face mask, and hyperbaric oxygen therapy [1,5]. Hyperbaric oxygen therapy is the adjunct therapy for cerebral air embolism by removing the volume of air embolus and improving the tissue oxygenation. The side effects of hyperbaric oxygen therapy are rare but can occur by oxygen toxicity on the cardiovascular and neurological systems. Hyperoxic convulsions are exceptionally rare and due to the reperfusion phenomenon.
Iatrogenic gas embolism is a serious disease, with a crude mortality rate of 25/119 (21%) at 1 year [11]. Therefore, cerebral air embolism should be treated as soon as possible, and immediate therapy has been reported to decrease the mortality rate to 7% [12].
To the best of our knowledge, this is the first case of status epilepticus caused by cerebral air embolism after the Valsalva maneuver. In addition, this report emphasizes the need for awareness of suspected cerebral air embolism following routine medical procedures to avoid poor prognosis.
REFERENCES
1. Bou-Assaly W, Pernicano P, Hoeffner E. Systemic air embolism after transthoracic lung biopsy: a case report and review of literature. World J Radiol. 2010; 2:193–6.

2. Green BT, Tendler DA. Cerebral air embolism during upper endoscopy: case report and review. Gastrointest Endosc. 2005; 61:620–3.

4. Lin C, Barrio GA, Hurwitz LM, Kranz PG. Cerebral air embolism from angioinvasive cavitary aspergillosis. Case Rep Neurol Med. 2014; 2014:406106.

5. Akhtar N, Jafri W, Mozaffar T. Cerebral artery air embolism following an esophagogastroscopy: a case report. Neurology. 2001; 56:136–7.

6. Jain KK. Textbook of hyperbaric medicine. 5th ed. Cambridge: Hogrefe;2009. p. 578.
7. Kim SK, Jun IG, Jang DM, Lim J, Hwang GS, Kim YK. Cerebral air embolism and subsequent transient neurologic abnormalities in a liver transplant recipient following the removal of the pulmonary artery catheter from the central venous access device: a case report. Korean J Anesthesiol. 2016; 69:80–3.

8. Schlimp CJ, Loimer T, Rieger M, Lederer W, Schmidts MB. The potential of venous air embolism ascending retrograde to the brain. J Forensic Sci. 2005; 50:906–9.

9. van Hulst RA, Klein J, Lachmann B. Gas embolism: pathophysiology and treatment. Clin Physiol Funct Imaging. 2003; 23:237–46.

10. Finsterer J, Stöllberger C, Bastovansky A. Cardiac and cerebral air embolism from endoscopic retrograde cholangio-pancreatography. Eur J Gastroenterol Hepatol. 2010; 22:1157–62.

Fig. 1.
(A) Brain computed tomography 30 minutes after symptom onset; multiple air bubbles observed prominently in the right parenchymal and subarachnoid vessels (arrowheads) . (B, C) Brain magnetic resonance imaging after 5 days after symptom onset; diffuse leptomeningeal enhancement of the right frontal, bilateral parietooccipital, and bilateral cerebellum with diffuse enhancement in the perivascular space of deep white matter(arrows).




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download