This article has been
cited by other articles in ScienceCentral.
Abstract
Background
A few cases of moyamoya syndrome associated with thyrotoxicosis have been reported. However, studies on the association of hyperthyroidism with moyamoya syndrome are insufficient.
Case Report
Here we report a case of hyperthyroidism associated with moyamoya syndrome in a 41-year-old woman with aphasia and right side weakness. Brain imaging revealed acute cerebral infarction of left middle cerebral artery territory and occlusion of bilateral distal internal carotid arteries.
Conclusion
Antithyroid medication stabilized the patient’s neurologic deterioration, suggesting that thyrotoxicosis could aggravate acute cerebral infarction caused by moyamoya syndrome.
Go to :

Keywords: Moyamoya disease, Thyrotoxicosis, Cerebral infarction
INTRODUCTION
The moyamoya syndrome is a cerebrovascular disease that predisposes patients to stroke due to progressive stenosis of the intracranial internal carotid arteries and their proximal branches [
1,
2]. Patients who have both characteristic moyamoya vasculopathy and well-recognized associated conditions are said to have the moyamoya syndrome, whereas patients with no known associated conditions are categorized as having moyamoya disease (MMD).
The well-recognized associated conditions are Down syndrome, sickle cell anemia, neurofibromatosis and rarely hyperthyroidism, known as Grave’s disease. However, studies on the association of hyperthyroidism with moyamoya syndrome are still lacking. We report a case of acute cerebral infarction in a patient with moyamoya syndrome which appeared to be aggravated by thyrotoxicosis.
Go to :

CASE REPORT
A 41-year-old woman visited the emergency room with aphasia, which had developed 10 hours earlier. The patient was diagnosed with thyrotoxicosis 2 years ago and treated with methimazole. The patient had no history of hypertension, diabetes, smoking or cerebral infarction or cerebral hemorrhage. Blood pressure at the time of admission was 165/78 mmHg, and heart rate was 123 times per minute. On physical examination, goiter and exopthalmos were observed. A neurological examination revealed incomplete global aphasia, mild dysarthria and motor weakness of right arm, grade IV+ of Medical Research Council grade. Her initial score on the National Institutes of Health Stroke Scale (NIHSS) was 3.
Diffusion-weighted imaging confirmed acute cerebral infarction on left middle cerebral artery territory (
Fig. 1A) with low signal on apparent diffusion coefficient (
Fig. 1B). An axial T1-weighted magnetic resonance (MR) image did not exhibit the typical punctuate and curvilinear flow voids of the hypertrophied moyamoya collateral in the basal ganglia. However, a T1-weighted images with gadolinium enhancement and fluid-attenuated inversion recovery images revealed prominent leptomeningeal enhancement in the both hemispheres (
Fig. 1C,
D). It indicated leptomeningeal collateral blood flow called “ivy signs” which is useful for the diagnosis of MMD. MR angiography including the aortic arch showed total occlusion of bilateral distal internal carotid arteries (
Fig. 1E,
F) [
3,
4]. Moderate decreased perfusion in left frontotemporal lobe and right temporal lobe was detected in both basal imaging and more distinctly in post-zoladin injection imaging on single photon emission computed tomography (SPECT) (
Fig. 2).
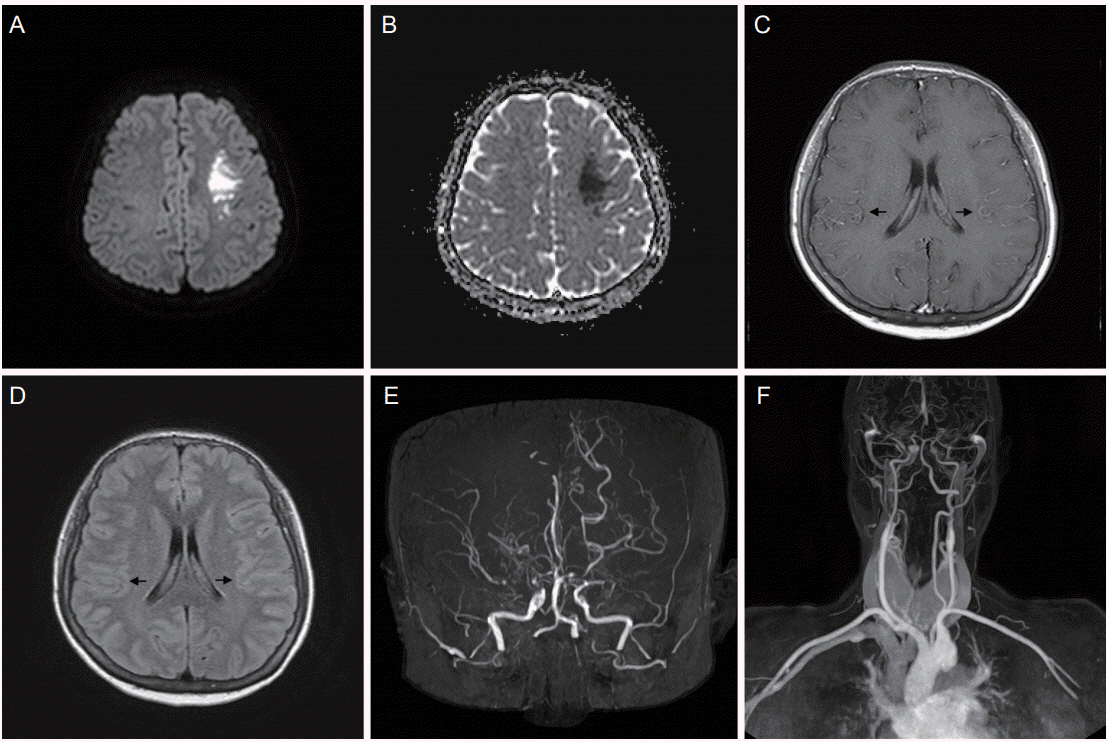 | Figure 1.Brain DWI, ADC, T1WI with gadolinium enhancement, FLAIR images, and head and neck MR angiographic images (A-F). DWI shows acute cerebral infarction on left middle cerebral artery territory (A) with low signal on ADC (B). T1WI images with gadolinium enhancement reveals prominent leptomeningeal enhancement in bilateral hemispheres (black arrows, C). FLAIR images show high signal intensity in the same area (black arrows, D). MR angiographic imaging reveals total occlusion of bilateral distal internal carotid arteries (E, F). DWI, diffusion-weight imaging; ADC, apparent diffusion coefficient; T1WI, T1-weighted images; FLAIR, fluidattenuated inversion recovery; MR, magnetic resonance. 
|
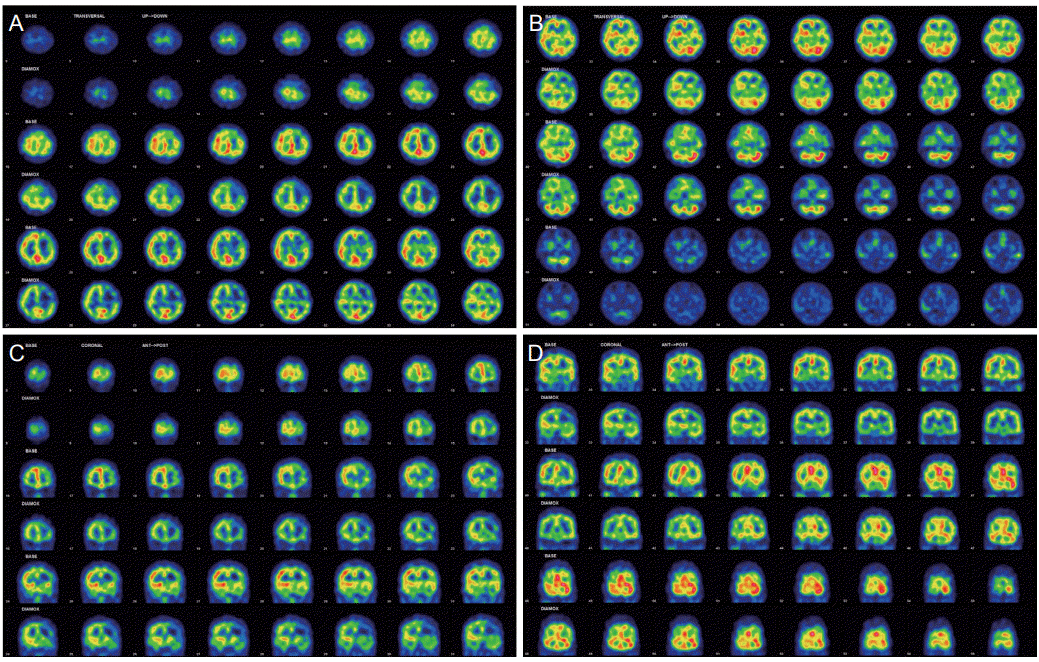 | Figure 2.Brain single photon emission computed tomography (A-D). Odd lines show basal imaging and even lines show post-zoladin imaging. These imaging confirmed decreased perfusion and collateral blood reservoir lacking in left frontotemporal lobe and right temporal lobe. 
|
In thyroid function test, T3 was increased to 523 ng/dL (reference, 65-150 ng/dL). Free T4 was increased to 9.08 ng/dL (reference, 0.89-1.76 ng/dL). Thyroid stimulating hormone (TSH) was decreased to 0.01 μIU/mL (reference, 0.35-5.5 μIU/mL). Anti-TSH receptor antibody exceeded 40.00 IU/L (reference, 0-1.75 IU/L). In thyroid ultrasound, the thyroid was enlarged diffusely. Transthoracic echo was normal and there was no atrial fibrillation on 72-hour Holter monitoring. In other blood tests likely fluorescent antinuclear antibody, anti-neutrophil cytoplasmic antibody, there were no results suggestive of vasculitis or coagulopathy.
On the second day of admission, the patient’s aphasia and right side weakness deteriorated further. Then, her NIHSS was checked to 10. After the administration of methimazole and Lugol’s solution, the patient remained stable and there was no further symptom deterioration. At the end of 2 weeks, the patient’s NIHSS was 5 and thyroid function test except for TSH was normalized.
Go to :

DISCUSSION
A few cases of moyamoya syndrome associated with thyrotoxicosis have been reported. Most hypotheses suggest the involvement of hyperkinetic metabolism including tachycardia, high pulse pressure, and decreased peripheral resistance secondary to vasorelaxation in moyamoya syndrome. Cardiovascular hemodynamic changes and increased atrial fibrillation are associated with cerebral thromboembolism [
5,
6]. Grave’s disease increases sympathetic nervous system sensitivity which may induce pathological changes of arterial walls [
7]. T-cell dysregulation of Grave’s disease is related to cellular proliferation and vascular dysregulation in moyamoya syndrome [
8]. In addition, hyperhomocysteinemia due to Grave’s disease is associated with premature atherosclerosis and moyamoya syndrome [
6].
However, it remains unclear why ischemic events occur during thyrotoxicosis in vasodilated patients. Detailed description about the mechanism is insufficient. Recently, a few case reports suggest that coronary artery spasm is the mechanism of myocardial ischemia secondary to thyrotoxicosis [
9-
11]. Among a total of 192 patients with coronary angiogram, free T4 levels were associated with coronary artery stenosis [
10]. The reason why patients with thyrotoxicosis are more prone to develop vascular spasm is currently unknown. However, that study suggests that, even within normal range, a small increase of free T4 level could be associated with coronary artery disease [
10].
In addition, vascular responses are enhanced by the sensitivity of endothelial components. Hyperthyroidism causes significant basal vasodilation due to excessive production of endothelial nitric oxide [
12]. In patients with moyamoya syndrome, brain SPECT after injection of acetazolamide which induces vasodilation could show intracerebral steal phenomenon, indicating that the collateral vascular area has already been expanded to its maximum extent.
This view might be applicable when taking CT angiography with contrast agents in those who are likely to have poor vascular reservoir in acute stroke patients. Excess iodine intake can induce transient or permanent hyperthyroidism in susceptible patients. Hyperthyroidism can be caused by quiescent nodules that become hyperfunctioning when they are exposed to excess iodine. Risk factors for hyperthyroidism include nontoxic diffuse or nodular goiter, latent Graves’ disease, and long-standing iodine deficiency [
13].
In conclusion, the present case appears to show the association of thyrotoxicosis with acute cerebral infarction in moyamoya syndrome. This is supported by the absence of other triggering factors and the improvement of neurologic deficits after antithyroid drug therapy. Therefore, physicians should consider thyroid function and Graves’ disease for acute ischemic stroke patients with moyamoya syndrome.
Go to :

ACKNOWLEDGMENTS
This research was supported by Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Education (NRF2017R1D1A1B03029672).
Go to :

REFERENCES
1. Scott RM, Smith ER. Moyamoya disease and moyamoya syndrome. N Engl J Med. 2009; 360:1226–37.

2. Kim JS. Moyamoya disease: epidemiology, clinical features, and diagnosis. J Stroke. 2016; 18:2–11.

3. Lee JY, Kim SK. Update of moyamoya disease. J Korean Med Assoc. 2007; 50:1109–18.

4. Seo KD, Suh SH, Kim YB, Kim JH, Ahn SJ, Kim DS, et al. Ivy sign on fluid-attenuated inversion recovery images in moyamoya disease: correlation with clinical severity and old brain lesions. Yonsei Med J. 2015; 56:1322–7.

5. Polikar R, Burger AG, Scherrer U, Nicod P. The thyroid and the heart. Circulation. 1993; 87:1435–41.

6. Napoli R, Biondi B, Guardasole V, Matarazzo M, Pardo F, Angelini V, et al. Impact of hyperthyroidism and its correction on vascular reactivity in humans. Circulation. 2001; 104:3076–80.

7. Ohba S, Nakagawa T, Murakami H. Concurrent Graves’ disease and intracranial arterial stenosis/occlusion: special considerations regarding the state of thyroid function, etiology, and treatment. Neurosurg Rev. 2011; 34:297–304. discussion 304.

8. Tendler BE, Shoukri K, Malchoff C, MacGillivray D, Duckrow R, Talmadge T, et al. Concurrence of Graves’ disease and dysplastic cerebral blood vessels of the moyamoya variety. Thyroid. 1997; 7:625–9.

9. Kim SJ, Heo KG, Shin HY, Bang OY, Kim GM, Chung CS, et al. Association of thyroid autoantibodies with moyamoyatype cerebrovascular disease: a prospective study. Stroke. 2010; 41:173–6.
10. Jung CH, Rhee EJ, Shin HS, Jo SK, Won JC, Park CY, et al. Higher serum free thyroxine levels are associated with coronary artery disease. Endocr J. 2008; 55:819–26.

11. Choi YH, Chung JH, Bae SW, Lee WH, Jeong EM, Kang MG, et al. Severe coronary artery spasm can be associated with hyperthyroidism. Coron Artery Dis. 2005; 16:135–9.

12. Al Jaber J, Haque S, Noor H, Ibrahim B, Al Suwaidi J. Thyrotoxicosis and coronary artery spasm: case report and review of the literature. Angiology. 2010; 61:807–12.

13. Lee SY, Rhee CM, Leung AM, Braverman LE, Brent GA, Pearce EN. A review: radiographic iodinated contrast media-induced thyroid dysfunction. J Clin Endocrinol Metab. 2015; 100:376–83.

Go to :






 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download