Introduction
Materials and Methods
1. Patient population
2. Treatment
3. 18F-FDG-PET/CT
4. PET metrics
5. Follow-up
6. Statistical analysis
Results
1. Patients
Table 1
| Total (n=116) | |
|---|---|
| Patient and tumor characteristic | |
| Age (yr) | 55 (48–64) |
| Pathology | |
| Squamous cell carcinoma | 99 (85.3) |
| Adenocarcinoma | 16 (13.8) |
| Adenosquamous carcinoma | 1 (0.9) |
| Tumor size (cm) | 5.1 (4.0–6.3) |
| ≤ 4 | 30 (25.9) |
| > 4, ≤ 6 | 55 (47.4) |
| > 6 | 31 (26.7) |
| Pelvic lymph node involvement | 86 (74.1) |
| Retroperitoneal lymph node involvement | 28 (24.1) |
| FIGO stagea) | |
| I | 2 (1.7) |
| II | 25 (21.6) |
| III | 81 (69.8) |
| IV | 8 (6.9) |
| Treatment characteristics | |
| HRCTV (cm3) | 51.7 (34.0–81.1) |
| Total (EBRT+IGBT) D90 HRCTV (Gy) | 77.9 (75.5–81.2) |
| Metabolic parameter | |
| PETbaseSUVmax | 13.4 (10.4–17.6) |
| PETbaseSUVmean | 6.4 (5.1–8.5) |
| PETbaseMTV (mL) | 40.4 (18.9–64.7) |
| PETbaseTLG | 264.9 (110.0–544.6) |
| PETIGBTSUVmax | 5.0 (3.5–6.6) |
| PETIGBTSUVmean | 2.7 (2.2– 3.3) |
| PETIGBTMTV (mL) | 7.8 (3.4–14.2) |
| PETIGBTTLG | 20.2 (7.6–43.2) |
| ΔSUVmax (%) | 65.4 (50.3–73.0) |
| ΔSUVmean (%) | 60.5 (48.1–66.9) |
| ΔMTV (%) | 78.3 (64.5–88.5) |
| ΔTLG (%) | 92.9 (84.2–96.0) |
Values are presented as number (%) or median (interquartile range). D90 HRCTV, biologically equivalent dose in 2Gy fractions to 90% of HRCTV (α/β of 10); EBRT, external beam radiation therapy; FDG-PET/CT, 18F-fluorodeoxyglucose positron emission tomography/computed tomography; FIGO, Fédération Internationale de Gynécologie et d’Obstétrique; HRCTV, high-risk clinical target volume; IGBT, image-guided brachy-therapy; MTV, metabolic tumor volume; PETbase, baseline FDG-PET/CT; PETIGBT, image-guided brachytherapy planning FDGPET/CT; SUVmax, maximum standardized uptake value; SUVmean, mean standardized uptake value; TLG, total lesion glycolysis.
2. PET metrics
Fig. 1
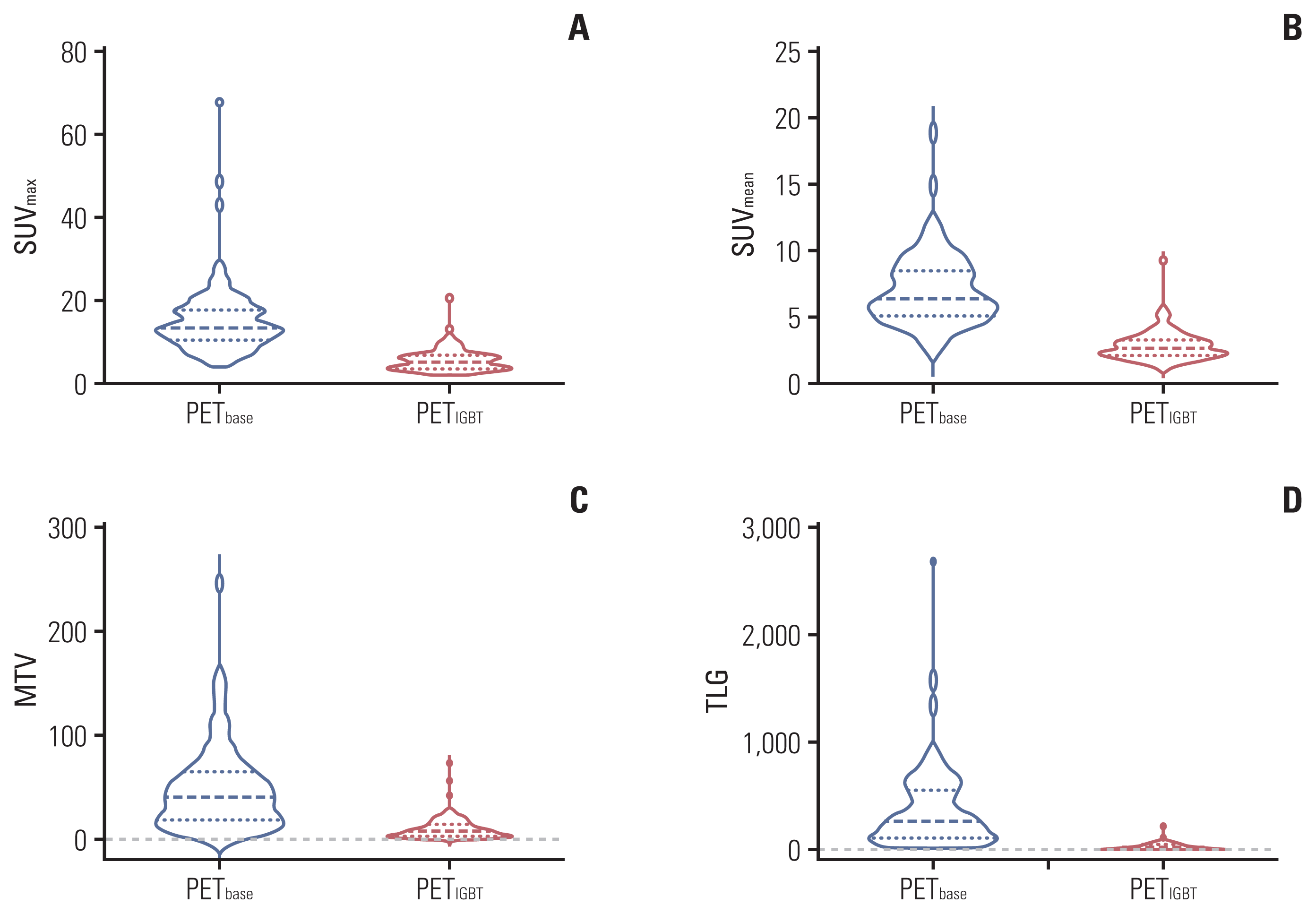
3. Treatment outcomes
Fig. 2
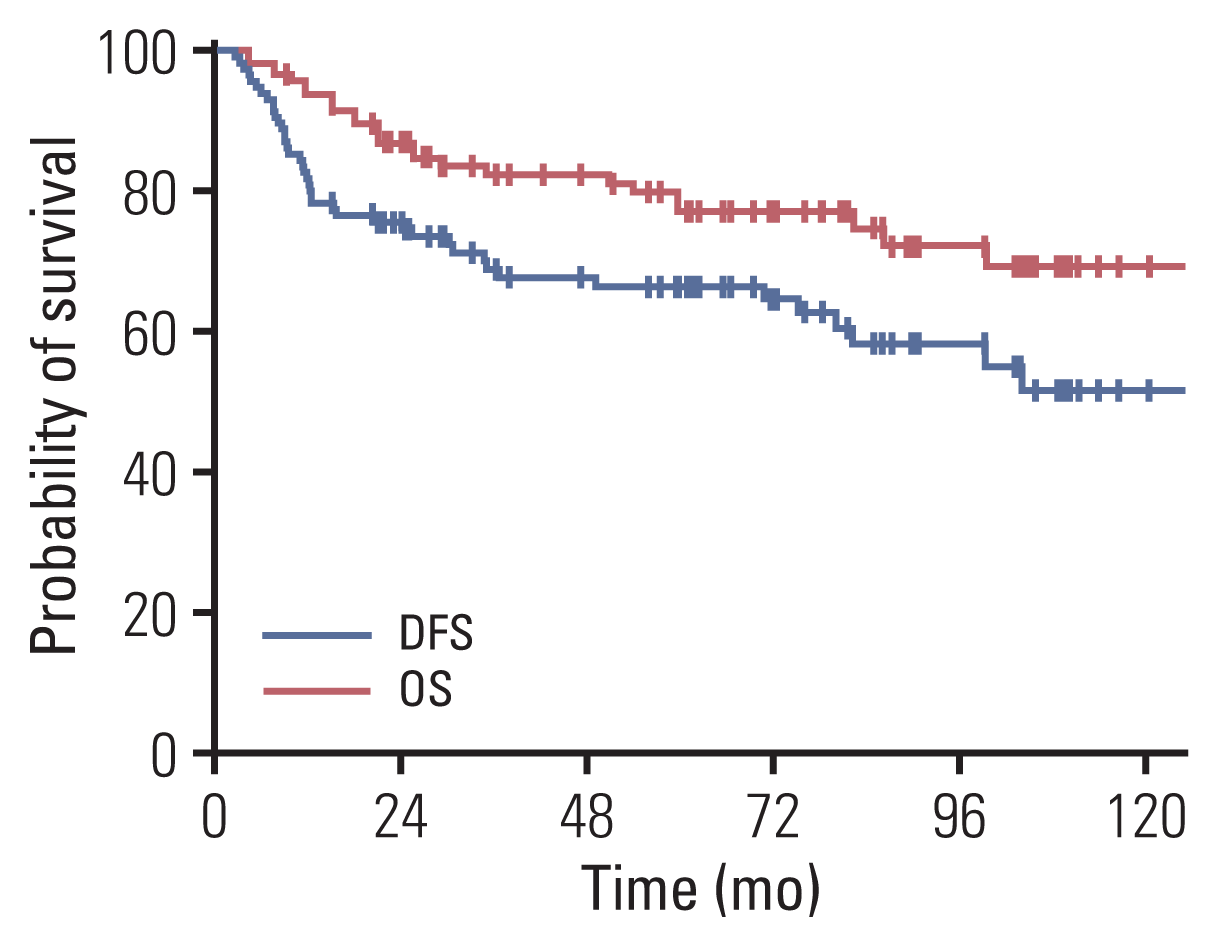
Table 2
| Variable | Univariable analysis | Multivariable analysis | ||||
|---|---|---|---|---|---|---|
| HR | 95% CI | p-value | HR | 95% CI | p-value | |
| Disease-free survival | ||||||
| Age (continuous) | 0.99 | 0.97–1.02 | 0.507 | - | - | - |
| Pathology (SCC vs. ADC/AD-SC) | 3.56 | 1.81–6.98 | < 0.001 | 2.98 | 1.38–6.48 | 0.006 |
| FIGO stagea) (I–II vs. III–IV) | 3.25 | 1.16–9.11 | 0.025 | 4.16 | 1.31–13.19 | 0.016 |
| Overall treatment time (≤ 56 days vs. > 56 days) | 1.26 | 0.58–2.73 | 0.566 | - | - | - |
| Chemotherapy regimen (cisplatin vs. cisplatin/5-FU) | 1.11 | 0.64–1.93 | 0.701 | - | - | - |
| HRCTV (< 50 cm3 vs. ≥ 50 cm3) | 2.17 | 1.13–4.19 | 0.021 | 1.00 | 0.99–1.01 | 0.657 |
| D90 HRCTV (≥ 78 Gy vs. < 78 Gy) | 1.39 | 0.76–2.54 | 0.293 | - | - | - |
| Tumor size (cm) | ||||||
| ≤ 4 vs. 4–6 | 1.17 | 0.51–2.69 | 0.709 | - | - | - |
| ≤ 4 vs. > 6 | 2.07 | 0.88–4.90 | 0.096 | - | - | - |
| PETbaseSUVmax (< 9 vs. ≥ 9) | 2.11 | 0.75–5.92 | 0.155 | - | - | - |
| PETbaseSUVmean (< 7 vs. ≥ 7) | 0.57 | 0.30–1.08 | 0.084 | - | - | - |
| PETbaseMTV (< 40 mL vs. ≥ 40 mL) | 2.23 | 1.18–4.23 | 0.014 | 1.81 | 0.86–3.79 | 0.118 |
| PETbaseTLG (< 144 vs. ≥ 144) | 1.03 | 1.00–1.07 | 0.073 | - | - | - |
| PETIGBTSUVmax (< 5 vs. ≥ 5) | 2.16 | 1.15–4.06 | 0.016 | 0.60 | 0.21–1.71 | 0.342 |
| PETIGBTSUVmean (< 3 vs. ≥ 3) | 1.98 | 1.08–3.61 | 0.026 | 1.57 | 0.61–4.10 | 0.351 |
| PETIGBTMTV (< 9 mL vs. ≥ 9 mL) | 2.19 | 1.19–4.03 | 0.012 | 1.38 | 0.67–2.83 | 0.386 |
| PETIGBTTLG (< 30 vs. ≥ 30) | 1.44 | 1.13–1.83 | 0.004 | 0.99 | 0.93–1.05 | 0.762 |
| ΔSUVmax (≥ 50% vs. < 50%) | 4.10 | 2.21–7.60 | < 0.001 | 2.56 | 1.14–5.77 | 0.023 |
| ΔSUVmean (≥ 60% vs. < 60%) | 3.30 | 1.69–6.44 | < 0.001 | 2.16 | 0.95–4.87 | 0.065 |
| ΔMTV (≥ 85% vs. < 85%) | 1.51 | 0.77–2.95 | 0.231 | - | - | - |
| ΔTLG (≥ 95% vs. < 95%) | 1.02 | 0.88–1.16 | 0.937 | - | - | - |
| Overall survival | ||||||
| Age (continuous) | 1.01 | 0.98–1.04 | 0.467 | - | - | - |
| Pathology (SCC vs. ADC/AD-SC) | 3.82 | 1.69–8.61 | 0.001 | 2.65 | 1.14–6.16 | 0.024 |
| FIGO stagea) (I–II vs. III–IV) | 1.69 | 0.58–4.90 | 0.337 | - | - | - |
| Overall treatment time (≤ 56 days vs. > 56 days) | 2.33 | 0.97–5.60 | 0.060 | - | - | - |
| Chemotherapy regimen (cisplatin vs. cisplatin/5-FU) | 0.86 | 0.42–1.74 | 0.665 | - | - | - |
| HRCTV (< 50 cm3 vs. ≥ 50 cm3) | 2.02 | 0.88–4.65 | 0.100 | - | - | - |
| D90 HRCTV (≥ 78 Gy vs. < 78 Gy) | 1.33 | 0.61–2.90 | 0.473 | - | - | - |
| Tumor size (cm) | ||||||
| ≤ 4 vs. 4–6 | 0.84 | 0.31–2.27 | 0.724 | - | - | - |
| ≤ 4 vs. > 6 | 1.40 | 0.50–3.96 | 0.520 | - | - | - |
| PETbaseSUVmax (< 9 vs. ≥ 9) | 1.13 | 0.39–3.30 | 0.816 | - | - | - |
| PETbaseSUVmean (< 7 vs. ≥ 7) | 0.54 | 0.23–1.23 | 0.143 | - | - | - |
| PETbaseMTV (< 40 mL vs. ≥ 40 mL) | 1.56 | 0.71–3.45 | 0.268 | - | - | - |
| PETbaseTLG (< 144 vs. ≥ 144) | 1.05 | 0.96–1.04 | 0.964 | - | - | - |
| PETIGBTSUVmax (< 5 vs. ≥ 5) | 3.65 | 1.46–9.09 | 0.005 | 1.86 | 0.68–5.05 | 0.226 |
| PETIGBTSUVmean (< 3 vs. ≥ 3) | 2.45 | 1.11–5.41 | 0.026 | 1.26 | 0.44–3.25 | 0.378 |
| PETIGBTMTV (< 9 mL vs. ≥ 9 mL) | 1.61 | 0.74–3.49 | 0.227 | - | - | - |
| PETIGBTTLG (< 30 vs. ≥ 30) | 1.65 | 0.86–3.16 | 0.130 | - | - | - |
| ΔSUVmax (≥ 50% vs. < 50%) | 7.57 | 3.39–16.90 | < 0.001 | 5.14 | 1.55–17.01 | 0.007 |
| ΔSUVmean (≥ 60% vs. < 60%) | 3.75 | 1.50–9.35 | 0.005 | 1.03 | 0.28–3.73 | 0.965 |
| ΔMTV (≥ 85% vs. < 85%) | 1.54 | 0.65–3.66 | 0.331 | - | - | - |
| ΔTLG (≥ 95% vs. < 95%) | 2.72 | 1.42–5.22 | 0.003 | 2.55 | 1.00–6.54 | 0.051 |
The foreparts of the parentheses were set as the reference group. 5-FU, 5-fluorouracil; ADC, adenocarcinoma; AD-SC, adenosquamous carcinoma; CI, confidence interval; D90 HRCTV, biologically equivalent dose in 2 Gy fractions to HRCTV (α/β of 10); FDG-PET/CT, 18F-fluorodeoxyglucose positron emission tomography/computed tomography; FIGO, Fédération Internationale de Gynécologie et d’Obstétrique; HR, hazard ratio; HRCTV, high-risk clinical target volume; MTV, metabolic tumor volume; PETbase, baseline FDG-PET/CT; PETIGBT, image-guided brachytherapy planning FDG-PET/CT; SCC, squamous cell carcinoma; SUVmax, maximum standardized uptake value; SUVmean, mean standardized uptake value; TLG, total lesion glycolysis.
Fig. 3

Fig. 4
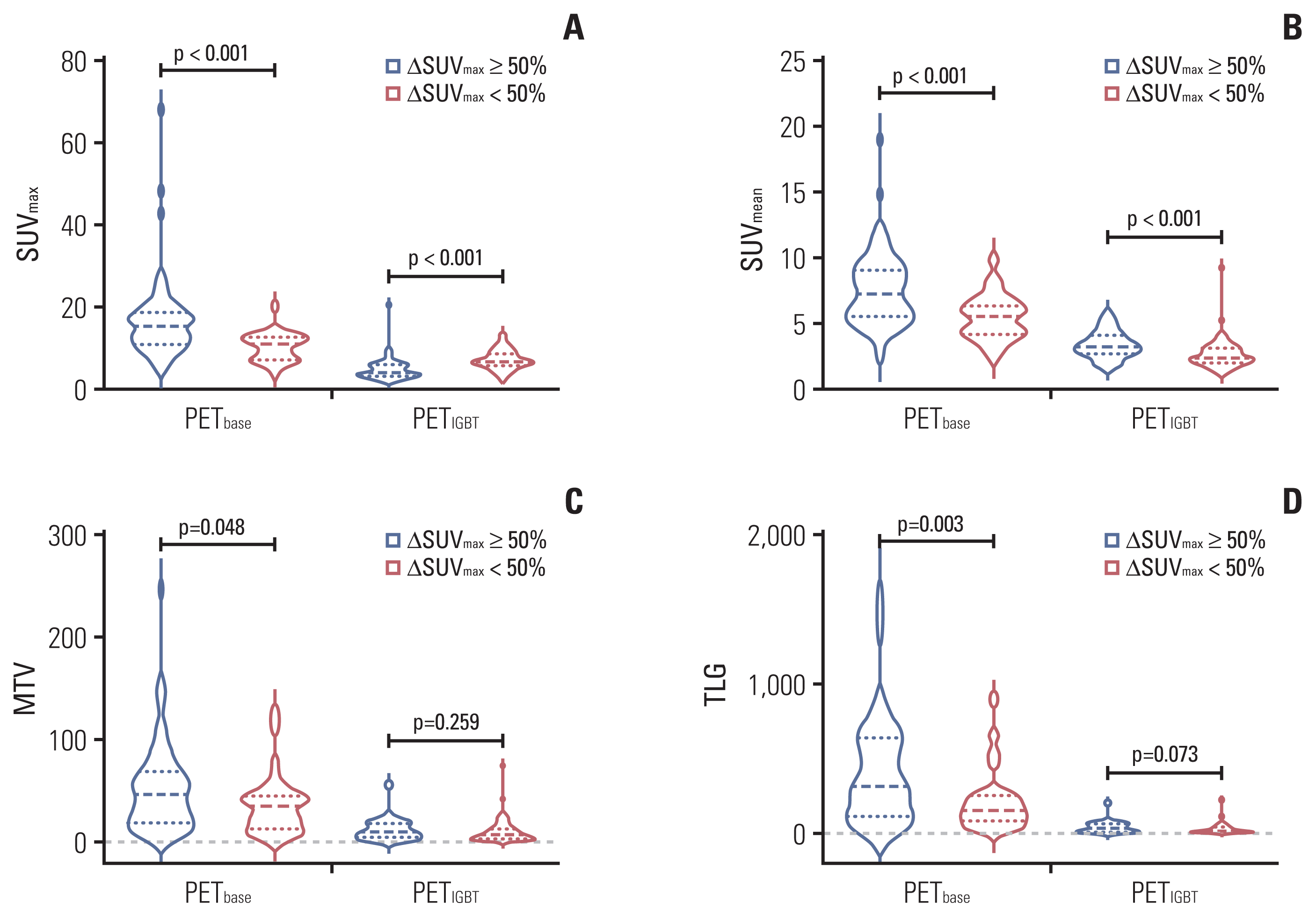
Table 3
| ΔSUVmax ≥ 50% (n=87) | ΔSUVmax < 50% (n=29) | p-value | |
|---|---|---|---|
| Patient and tumor characteristic | |||
| Age (yr) | 54 (48–63) | 56 (46–65) | 0.678 |
| Pathology | |||
| SCC | 78 (89.7) | 21 (72.4) | 0.033 |
| ADC/AD-SC | 9 (10.3) | 8 (27.6) | |
| FIGO stagea) | |||
| I | 2 (2.3) | 0 | 0.491 |
| II | 21 (24.1) | 4 (13.8) | |
| III | 59 (67.8) | 22 (75.9) | |
| IV | 5 (5.7) | 3 (10.3) | |
| Tumor size (cm) | 5.2 (4.2–6.4) | 5.0 (3.7–6.0) | 0.177 |
| ≤ 4 | 20 (23.0) | 10 (34.5) | 0.429 |
| > 4, ≤ 6 | 42 (48.3) | 13 (44.8) | |
| > 6 | 25 (28.7) | 6 (20.7) | |
| Pelvic lymph node involvement | 62 (71.3) | 24 (82.8) | 0.327 |
| Retroperitoneal lymph node involvement | 20 (23.0) | 8 (27.6) | 0.802 |
| Treatment characteristic | |||
| Total EBRT dose (Gy) | 50.4 (50.4–50.4) | 50.4 (50.4–50.4) | 0.745 |
| Total IGBT dose (Gy) | 24.0 (24.0–24.0) | 24.0 (24.0–24.0) | 0.420 |
| HRCTV (cm3) | 51.0 (33.6–74.2) | 60.3 (35.9–92.6) | 0.637 |
| Total (EBRT+IGBT) D90 HRCTV (Gy) | 78.3 (75.9–81.5) | 76.5 (75.0–79.4) | 0.092 |
| PET parameter | |||
| PETbaseSUVmax | 15.4 (11.1–18.8) | 11.0 (7.2–12.6) | < 0.001 |
| PETbaseSUVmean | 7.2 (5.6–9.0) | 5.6 (4.2–6.4) | < 0.001 |
| PETbaseMTV (mL) | 46.0 (20.3–68.3) | 35.1 (14.8–43.9) | 0.048 |
| PETbaseTLG | 311.7 (113.3–628.1) | 150.8 (90.2–240.3) | 0.003 |
| PETIGBTSUVmax | 4.2 (3.3–5.9) | 6.8 (5.9–8.8) | < 0.001 |
| PETIGBTSUVmean | 2.4 (2.1–3.1) | 3.3 (2.8–4.1) | < 0.001 |
| PETIGBTMTV (mL) | 7.1 (3.3–13.1) | 9.6 (4.7–18.3) | 0.259 |
| PETIGBTTLG | 17.4 (7.5–35.4) | 35.2 (11.6–61.2) | 0.073 |
| ΔSUVmax | 68.2 (61.9–75.2) | 33.7 (12.8–44.7) | < 0.001 |
| ΔSUVmean | 62.4 (56.8–70.9) | 41.6 (25.8–47.9) | < 0.001 |
| ΔMTV | 83.1 (70.8–89.1) | 58.4 (47.6–83.3) | 0.004 |
| ΔTLG | 93.6 (87.8–86.5) | 77.2 (61.2–93.0) | < 0.001 |
Values are presented as number (%) or median (interquartile range). ADC, adenocarcinoma; AD-SC, adenosquamous carcinoma; D90 HRCTV, biologically equivalent dose in 2 Gy fractions to 90% of HRCTV (α/β of 10); EBRT, external beam radiation therapy; FDG-PET/CT, 18F-fluorodeoxyglucose positron emission tomography/computed tomography; FIGO, Fédération Internationale de Gynécologie et d’Obstétrique; HRCTV, high-risk clinical target volume; IGBT, image-guided brachytherapy; MTV, metabolic tumor volume; PETbase, baseline FDG-PET/CT; PETIGBT, image-guided brachytherapy planning FDG-PET/CT; SCC, squamous cell carcinoma; SUVmax, maximum standardized uptake value; SUVmean, mean standardized uptake value; TLG, total lesion glycolysis.




 PDF
PDF Citation
Citation Print
Print


 XML Download
XML Download