Introduction
Materials and Methods
1. Search strategy and selection criteria
2. Data analysis
Results
Table 1
| Study | Study designe | No. of initial participants | Follow-up period (mo) | Age (yr), mean±SD or median (range) | Male, n (%) | Education (yr) | Cancer site | Cancer stage | Chemotherapy regimen | Neuropsychological measurement | Covariates | NOS score |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Main analysis | ||||||||||||
|
|
||||||||||||
| Andreis et al. (2013) [15] | Prospective study | 47a) | 6 | 58.68±9.62 | 16 (34.04) | 9.43 (3.91) | Colon (47) | 3 (47) | FOLFOX4 | oxaliplatin 980 (364), 5-FU 19,103 (6,840), 5-FU bolus 6,745 (2,431), 5-FU continuous infusion 14,075 (4,642), n. administration 11.42 (1.5) | Clock Drawing Test, Rey Auditory Verbal Learning Test (call/recall), Rey Complex Figure, (copy/recall) TMT A, TMT B, | Age, education, sex | 7 |
|
|
||||||||||||
| Vardy et al. (2019) (localized) [5] | Prospective study | 173 | 6 | 57.0 (23–75) | 117 (67.63) | 13.8 (3.3) | Colon (104), rectum (66) | 1 (2), 2 (46), 3 (125) | Adjuvant (123), neoadjuvant (46), unknown (4) | FU (54), oxaliplatin (72), chemoradiation (44), missing (3) | Clinical NP Tests (Letter-Number test, Digit span test, Spatial Span test, HVLT total, HVLT delayed, BVMT total, BVMT delayed, | Age, sex, education, time between assessments, practice effect | 9 |
| Vardy et al. (2015) (metastatic) [5] | Prospective study | 73 | 6 | 55.5 (28–75) | 40 (54.79) | 13.7 (3.4) | Colon (54), rectum (16) | 3 (4), 4 (69) | None (1), FU (4), oxaliplatin (36), chemoradiation (2), irinotecan (20), other (2), missing (4) | Digit Symbol test, TMT A, TMT B), Cambridge Neuropsychological Test Automated Battery (CANTAB) | ||
|
|
||||||||||||
| Cruzado et al. (2014) [4] | Prospective study | 81 | 6 | 66.96±9.52 | 50 (61.73) | 6.9 (4.1) | - | 1 (28), 2 (53) | Oxaliplatin plus 5-FU/leucovorin (FOLFOX4) adjuvant CT regimen within 6 to 8 weeks post-surgery | no. of chemotherapy 11.80 (0.54), dosage of oxaliplatin mg/m2 1,003.20 (129), total dose of 5-FU mg/m2 21,690 (3,744) | TMT A, TMT B, Interference score of the Stroop Color and Word Test, Digit Symbol test, Verbal memory subtest of the Barcelona test (Immediate/Delayed memory), Luria Memory Words Test | Age, education, sex | 6 |
|
|
||||||||||||
| Sales et al. (2019) [16] | Prospective study | 47 | 12 | 61.1±8.8 | 30 (63.83) | 7.9 (3.9) | - | 2 (23), 3 (24) | 6 cycles (6 mo) of 5-FU, leucovorin with or without oxaliplatin | HVLT, BVMT, Digit span-forward, TMT A, TMT B, Digit symbol test, Digit span (backwards), Semantic verbal fluency (animals), Stroop C test, Phonemic verbal fluency | Age, sex, education, depressive symptoms at baseline | 8 |
|
|
||||||||||||
| Anstey et al. (2015) [13] | Prospective study | 20 | 48 | - | - | - | - | - | - | TMT A, TMT B, California Verbal Learning Test (Immediate/Delayed), Symbol-Digit-Modality test, Simple/choice reaction time, Verbal Fluency (F words, A words) | Unadjustedb) | 6 |
|
|
||||||||||||
| Mayrbaurl et al. (2016) [17] | Prospective study | 100 | 3 cyclesc) | 66.4±10.6 | 60 (60) | - | - | Advanced colorectal cancer |
First-line palliative 73 (FU FA oxaliplatin 16.7%, FU leucovorin 5.3%, FU FA irinotecan panitumumab 15.3%, FU FA oxaliplatin bevacizumab 12.5%) Second-line palliative 63 (FU FA irinotecan 18.3%, panitumumab 15%, FU FA irinotecan cetuximab 11.7%, FU FA oxaliplatin bevacizumab 10%) Third-line or more palliative 47 (panitumumab 17.6%, FU FA bevacizumab 17.6%, FU FA oxaliplatin 12.2%, FU FA oxaliplatin bevacizumab 9.5%) |
EORTC-QLQ C30 Cognitive functioning | - | 4 |
|
|
||||||||||||
| Lee et al. (2016) [14] | Prospective study | 56 | 6 cyclesc) | 59.5±11.5 | 31 (55.36) | None 8 elementary school 17 middle school 10 high school 11 college or more 10 (people) | Rectal (56) | 2 (16), 3 (40), 4 (9) | 6 cycles of FOLFOX | EORTC-QLQ C30 Cognitive functioning | - | 4 |
|
|
||||||||||||
| Tsunoda et al. (2010) [18] | Prospective study | 99 | 7 | 65±10 | 58 (58.59) | - | Colon (59), rectal (40) | 2 (49), 3 (50) | Oral uracil/tegafure dose 300 mg/m2/day, oral leucovorin dose of 75 mg/day on days 1–28, followed by a 7-day rest (35 days/cycle ×5 cycles) | EORTC-QLQ C30 Cognitive functioning | - | 5 |
|
|
||||||||||||
| Subsidiary analysis | ||||||||||||
|
|
||||||||||||
| Couwenberg et al. (2018) (LAR) [19] | Prospective study | 134 | 3 | 64 (38–83) | 93 (69.4) | - | Rectal (134) | cT2 (16), cT3 (104), cT4 (14), cN0 (16), cN1 (55), cN2 (63), cM0 (121), cM1 (12) M stage unknown (1) | - | EORTC-QLQ C30 Cognitive functioning | - | 6 |
|
|
||||||||||||
| Couwenberg et al. (2018) (APR) [19] | Prospective study | 119 | 3 | 66 (26–87) | 91 (76.5) | - | Rectal (119) | cT1 (1), cT2 (15), cT3 (83), cT4 (20), cN0 (21), cN1 (51), cN2 (47), cM0 (113), cM1 (4), M stage unknown (2) | - | EORTC-QLQ C30 Cognitive functioning | - | |
|
|
||||||||||||
| Souza et al. (2018) [20] | Prospective study | 29 | 3 | 50.8±11.4 | 11 (38) | - | Rectal (29) | T3, T4, N+ | 5-FU 350 mg/m2 (1st, 5th day) | EORTC-QLQ C30 Cognitive functioning | - | 5 |
|
|
||||||||||||
| Bencova et al. [21] (men) | Prospective study | 43 | - | range (54–76) | 43 | - | Rectal (43) | T3, T4 | 5-FU 350 mg/m2/day | EORTC-QLQ C30.3 Cognitive functioning | - | 3 |
|
|
||||||||||||
| Bencova et al. [21] (women) | Prospective study | 21 | - | - | - | - | Rectal (21) | T3, T4 | 5-FU 350 mg/m2/day | EORTC-QLQ C30.3 Cognitive functioning | - | |
5-FU, 5-fluorouracil; APR, abdominoperineal resection; BVMT, Brief Visuospatial Memory Test; CT, computed tomography; EORTC-QLQ C30, European Organisation for Research and Treatment of Cancer Quality of Life Questionnaire Core 30; FA, folinic acid; FOLFOX, 5-fluorouracil, leucovorin, oxaliplatin regimen; HVLT, Hopkins Verbal Learning Test; LAR, low anterior resection; NOS, Newcastle-Ottawa Scale; SD, standard deviation; TMT, Trail Making Test.
1. Study characteristics
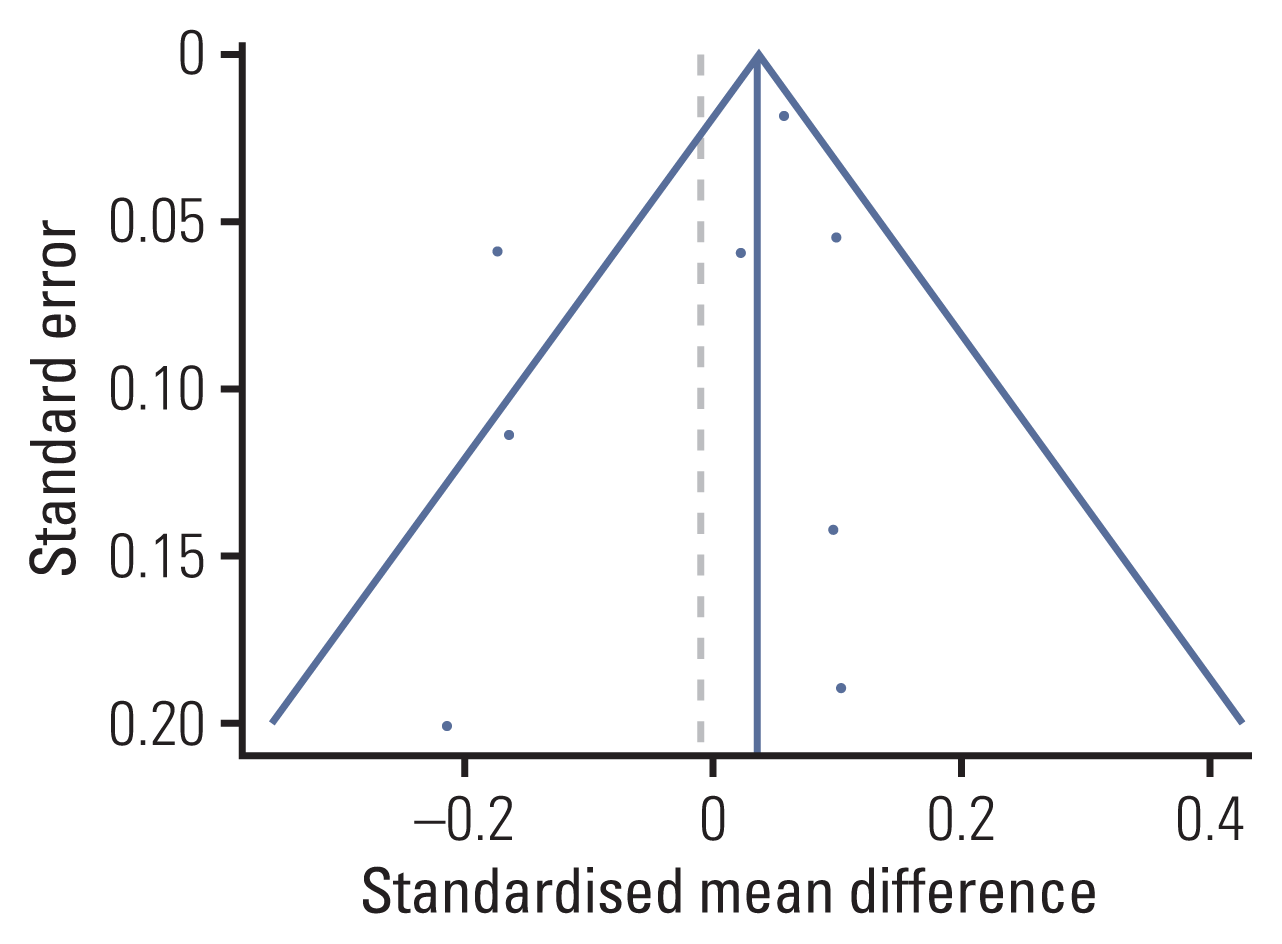
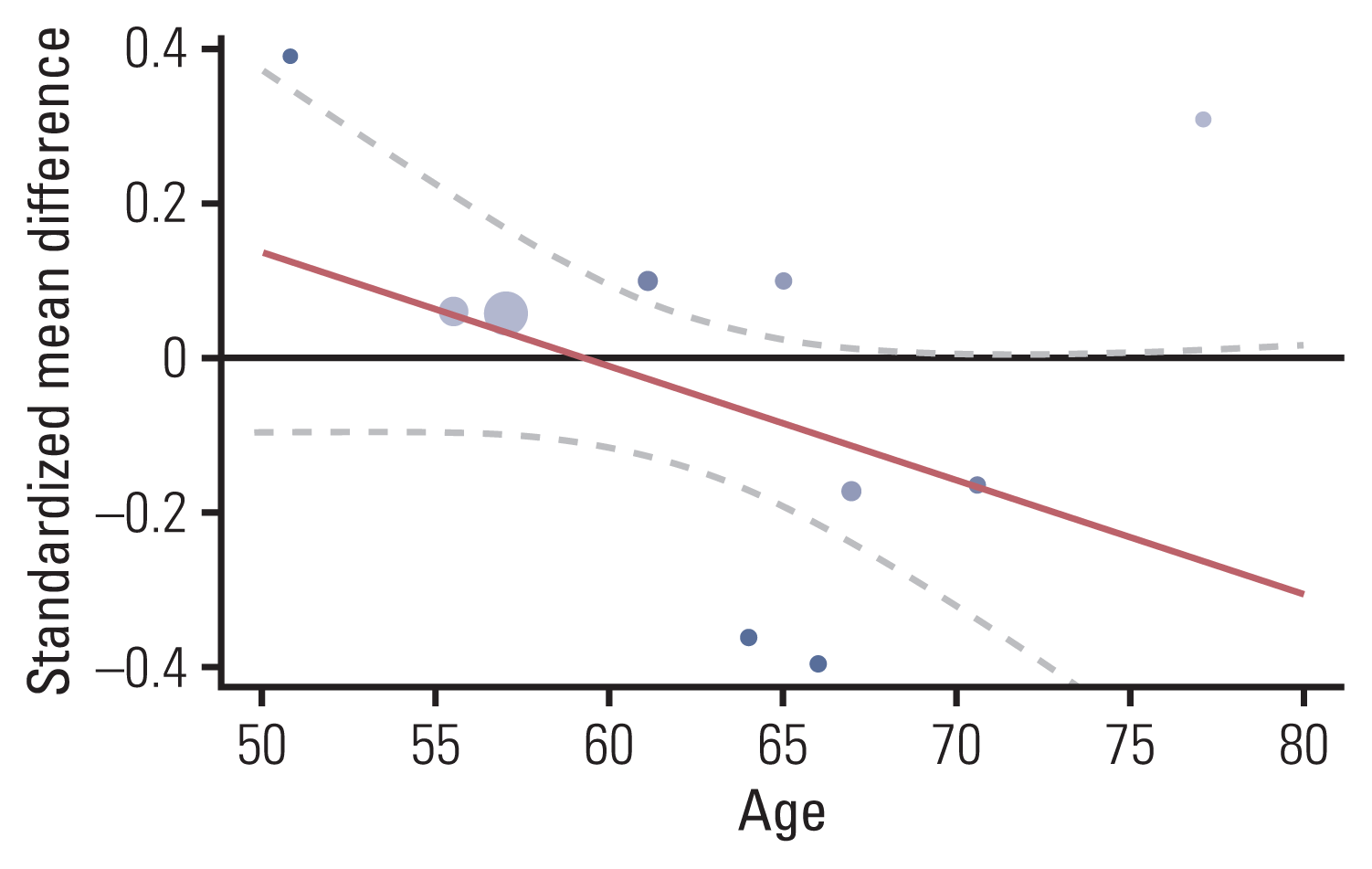
2. Effect sizes of overall cognitive function
Fig. 2
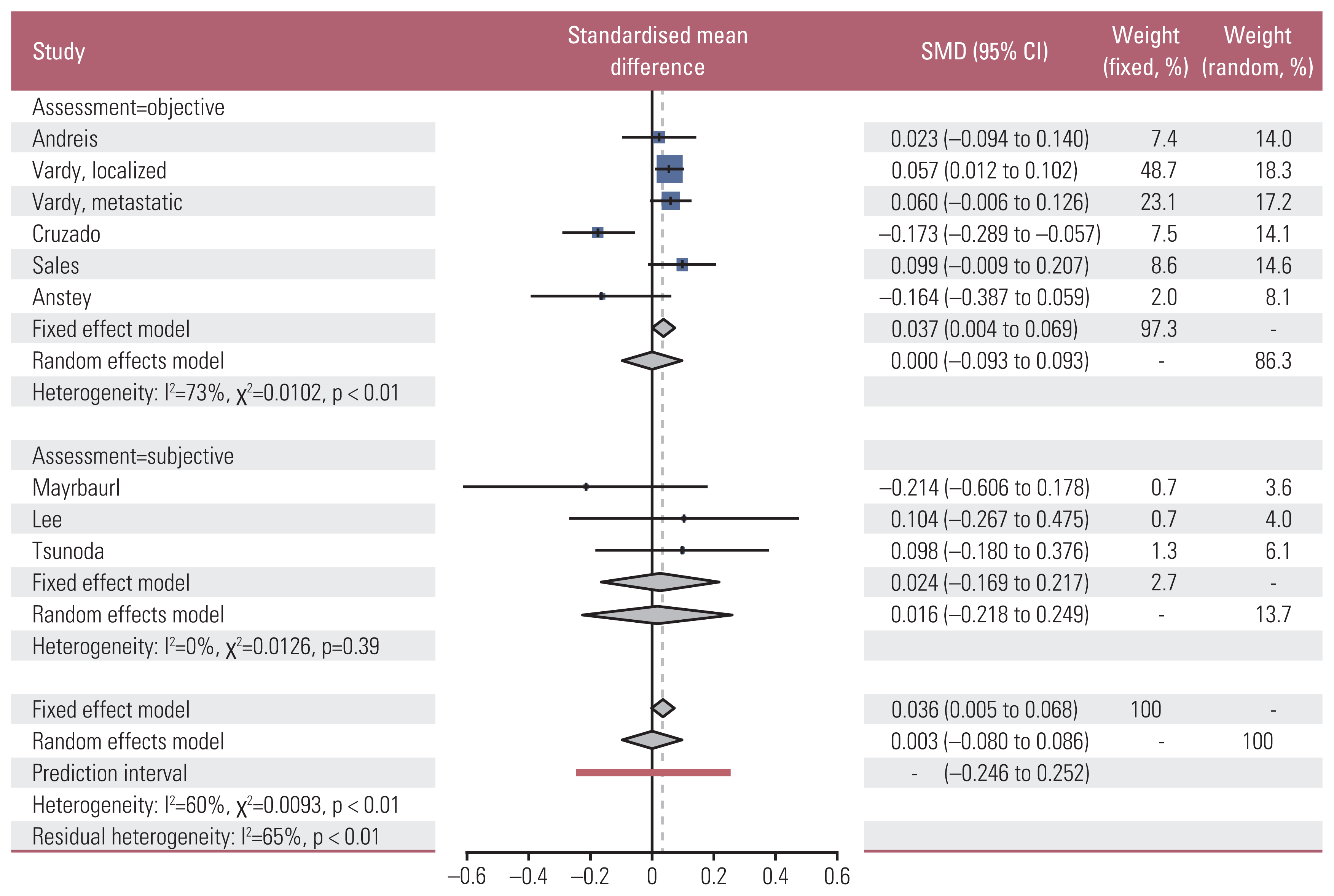
Table 2
| No. of initial participants | Follow-up (mo) | Male (%) | SMD | 95% CI | p-value | |
|---|---|---|---|---|---|---|
| Objective | ||||||
| Andreis et al. [15] | 47 | 6 | 34.0 | 0.023 | −0.094 to 0.140 | 0.697 |
| Vardy et al. [5], localized | 173 | 6 | 67.6 | 0.057 | 0.011 to 0.102 | 0.016 |
| Vardy et al. [5], metastatic | 73 | 6 | 54.8 | 0.060 | −0.006 to 0.126 | 0.075 |
| Cruzado et al. [4] | 81 | 6 | 61.7 | −0.173 | −0.289 to −0.057 | 0.003 |
| Sales et al. [16] | 47 | 12 | 63.8 | 0.099 | −0.009 to 0.207 | 0.074 |
| Anstey et al. [13] | 20 | 48 | N/A | −0.164 | −0.387 to 0.060 | 0.151 |
| Subtotal (I2=73%) | 441 | |||||
| Fixed | 0.037 | 0.004 to 0.069 | 0.026 | |||
| Random | 0.000 | −0.093 to 0.093 | 0.998 | |||
| Subjective | ||||||
| Mayrbaurl et al. [17] | 100 | 3 cyclesa) | 60.0 | −0.214 | −0.606 to 0.178 | 0.286 |
| Lee et al. [14] | 56 | 6 cyclesa) | 55.4 | 0.104 | −0.267 to 0.475 | 0.583 |
| Tsunoda et al. [18] | 99 | 7 | 58.6 | 0.098 | −0.169 to 0.217 | 0.324 |
| Subtotal (I2=0%) | 255 | |||||
| Fixed | 0.024 | −0.170 to 0.217 | 0.094 | |||
| Random | 0.015 | −0.219 to 0.249 | 0.601 | |||
| Total (I2=60%) | 696 | |||||
| Fixed | 0.036 | 0.005 to 0.068 | 0.025 | |||
| Random | 0.003 | −0.219 to 0.249 | 0.939 | |||




 PDF
PDF Citation
Citation Print
Print


 XML Download
XML Download