INTRODUCTION
Glanzmann thrombasthenia (GT) is a very rare and severe insufficiency of platelet function caused by an inherited deficiency or dysfunction of the glycoprotein IIb/IIIa complex and the platelet fibrinogen receptor [1]. Patients with GT have a tendency to develop spontaneous or post-traumatic bleeding. In GT patients, the operation itself is dangerous, and the risk of cerebral hemorrhage is considerably increased because of secondary brain damage.
CASE REPORT
A 45-year-old woman with a stuporous mentality visited the emergency department. She was unconscious, and it was hard to identify her medical history or family history. A brain computed tomographic (CT) angiography revealed a rupture of the posterior cerebral artery aneurysm. At the time of admission, her Hunt-Hess grade was 4 points and her Fisher grade was 3 points.
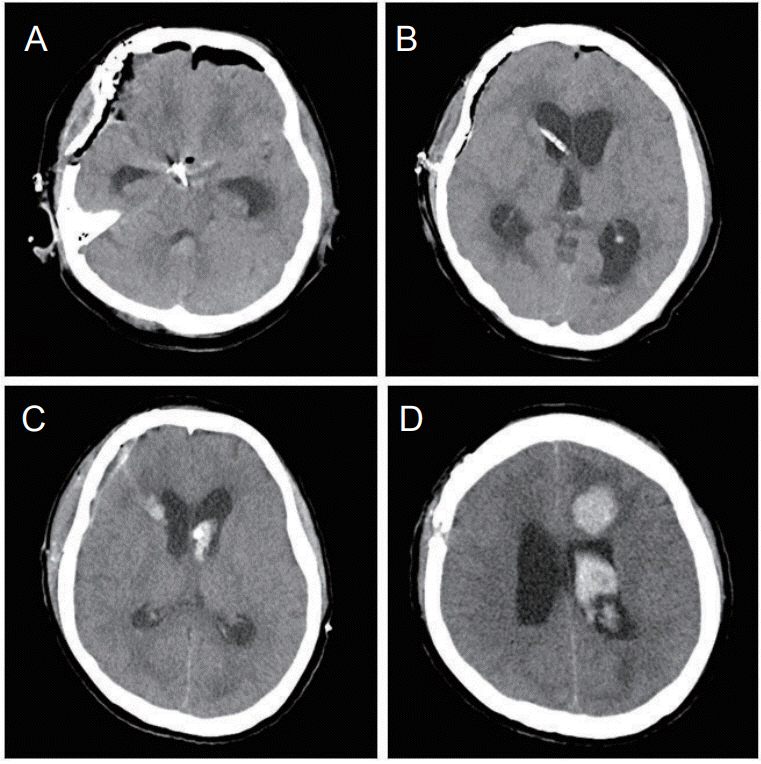
A clipping of the aneurysm was performed simultaneously with an insertion of a intraoperative external ventricular drainage (EVD) catheter for hydrocephalus (Fig. 1). After 7 days of the clip operation, a change of EVD catheter was performed, and a new intracranial hemorrhage was observed in each of the removal sites of the existing catheter in the right side and an insertion site of the new catheter in the left side (Fig. 1). An intracranial hemorrhage located along the EVD catheter identified by a CT scan was due to an iatrogenic injury during the procedure, not a rebleeding of the clipped aneurysm. In addition, the bleeding continued at the surgical wound of the scalp.
Various blood tests were conducted to evaluate the bleeding tendency, and most of the laboratory test results, including a coagulation factor assay and her Von Willebrand factor were normal. The hemoglobin was 13.5g/dL, hematocrit was 40%, platelet count was 288×103/μL, prothrombin time was 10.8 seconds, and activated partial thromboplastin time was 29 seconds. Additional tests were performed to determine the cause of the bleeding. A Platelet Function Analyzer-200 (PFA-200; Siemens, Munich, Germany) using a collagen+epinephrine embeded cartridges resulted in more than 254 seconds (normal range 81-192 seconds). The results of PFA-200 using a collagen+adenosine diphosphate embeded cartridges were prolonged to 210 seconds (normal range 61-116 seconds). Glanzmann thrombasthenia was confirmed by these blood test results, and also by the consulting of a hematologist. On the 25th postoperative day, she received a platelets transfusions for a ventriculoperitoneal shunt in close cooperation with an anesthesiologist and hematologist. After 50 days of hospitalization, she was discharged with a modified Rankin Scale score of 3 points and the Korean vision of Mini-Mental State Examination of 18 points. Due to her poor family relationships and cognitive function status, her past history could not be determined precisely. At the time of discharge, her caregiver was educated about how the bleeding of a minor injury may be able to be controlled by pressure or by taking a anti-fibrinolytic medicine.
DISCUSSION
Eduard Glanzmann was a Swiss pediatrician who first discovered the condition of thrombasthenia in 1918 [2]. Platelets are a principal constituent of many restorative physiological procedures, including hemostasis [3]. Acquired platelet diseases are more likely to be faced in clinical practice than their inherited counterparts, and often emerge as an effect from the onset of medications or underlying medical conditions. In contrast, inherited platelet disorders are rare, and until recently, have been under less scrutiny [3].
Bleeding events are variable among patients with Glanzmann’s thrombasthenia, and menorrhagia, epistaxis, and gingival bleeding are more frequent [4]. In a study by George et al. [5] on 113 patients in 1990, the less common Glanzmann’s thrombasthenia symptoms were gastroin-testinal bleeding (12%), hematuria (6%) and intracranial hemorrhage (ICH) (2%). Also, Toogeh et al. [6] in 2004, had reported that the most common clinical manifestation was epistaxis (49.7%) and central nervous system bleeding only occurred in one person (0.3%) of 382 patients with GT in Iran.
As it is known, congenital bleeding disorders are a rare manifestation but the leading cause of ICH, which has a high rate of morbidity and mortality [7]. There are two cases of in-utero death caused by intracranial hemorrhage, one at 24 weeks and the other at 31 weeks of gestation [8]. George et al. [5] reported two Paris patients in his review article. One patient had a meningeal hemorrhage after a head trauma at 4 years old, but less severe bleeding. The other patient had an intracerebral hematoma that required surgical evacuation after a fall at age 6, but the patient recovered well after surgery. The prevalence of ICH in Glanzmann’s thrombasthenia patients is extremely uncommon, but powerful enough to cause death [3,9].
Patients with GT do not require treatment on a regular basis, but will always need therapy during surgical procedures or a bleeding control after an injury [10]. Antifibrinolytic agents such as tranexamic acid may control the bleeding for mild to moderate events, but platelet transfusions as an alternative method must be considered when clinicians fail to control the bleeding [3]. Also, patients with GT should require registration with a 24-hour center capable of dealing with this kind of treatment [3,11,12].
If nonspecific and recurrent intracranial hemorrhage persists, GT should be included in the differential diagnosis, which is important in controlling and treating bleeding during the surgical procedures.




 PDF
PDF Citation
Citation Print
Print


 XML Download
XML Download