INTRODUCTION
The prevalence of intracranial aneurysm is 2% to 3% in the general population, and is more common in patients who experience acute ischemic stroke [1-3]. Intracranial aneurysms have been considered to be a relative contraindication to the administration of the recombinant tissue plasminogen activator (rtPA) due to the theoretical risk for an aneurysmal rupture in individuals who experience acute ischemic stroke [4]. However, recent studies have not reported an increase in the risk for rtPA-related intracranial hemorrhage in patients with both acute ischemic strokes and intracranial aneurysms [5-7]. In one prospective multicenter study, the authors provided reassurance that the risk for intracranial hemorrhage does not increase in acute ischemic stroke patients harboring an intracranial aneurysm [5]. The issue of administering rtPA to stroke patients with an intracranial aneurysm is very important because the evaluation of cerebral vessels to locate aneurysms may result in an in-hospital delay and exclude potential candidates for thrombolysis. Although international guidelines state that an intracranial aneurysm is a contraindication to rtPA, controversy remains as to whether an unruptured aneurysm should be regarded as a contraindication to rtPA, in patients who experience acute ischemic stroke.
To date, case reports describing the rupture of an intracranial aneurysm following the intravenous administration of rtPA are extremely rare and these studies have not convincingly demonstrated that rtPA causes a rupture of the aneurysm [8-10]. Hence, in routine clinical practice, rtPA tends to be emergently infused in patients exhibiting acute stroke symptoms when a non-contrast computed tomography (CT) detects no intracranial bleeding or any other remarkable contraindications to rtPA.
In this article, we describe a patient who developed aneurysmal subarachnoid hemorrhage (SAH) following the intravenous administration of rtPA for an acute ischemic stroke.
CASE REPORT
A 51-year-old man with untreated hypertension presented to the emergency room with speech disturbance, which had developed 30 minutes earlier. A neurological examination revealed global aphasia and right hemiparesis. His initial score on the National Institutes of Health Stroke Scale was 9. The standard protocol for acute ischemic stroke in the author’s hospital involves a non-contrast CT scan to exclude contraindications to rtPA. Then, after the administration of rtPA, a magnetic resonance imaging (MRI) and a magnetic resonance angiography are performed [11].
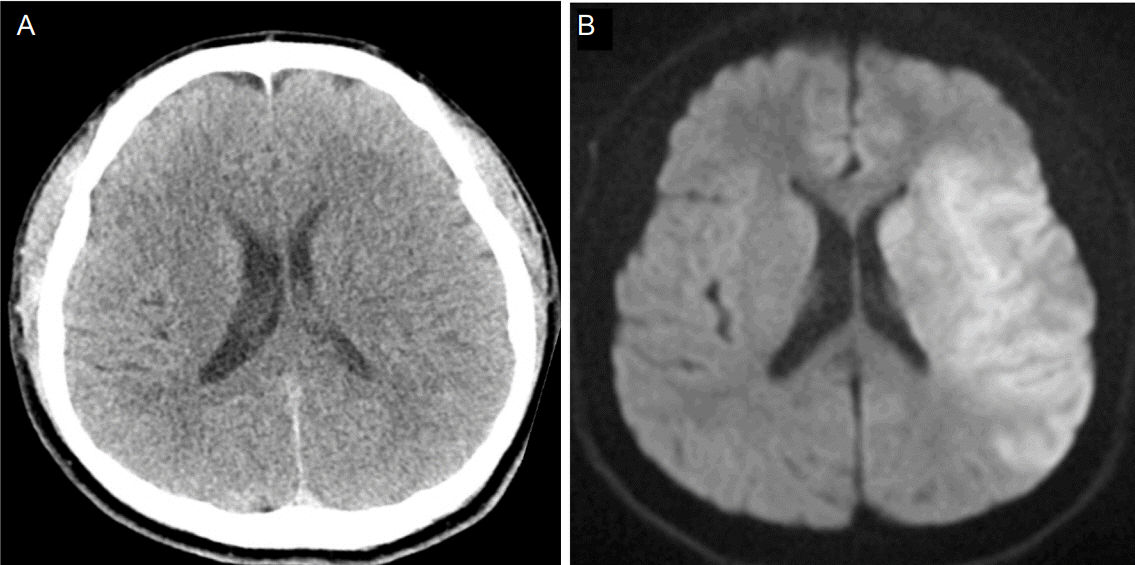
A CT scan of the patient’s brain did not reveal an intracranial hemorrhage (Fig. 1A). His initial blood pressure was 205/131 mmHg, but decreased to 162/75 mmHg, after the administration of intravenous labetalol. rtPA (0.9 mg/kg) was administered intravenously, 121 minutes after the onset of stroke symptoms. Diffusion-weighted imaging of the brain, which was performed during the infusion of rtPA, revealed acute infarction in the left middle cerebral artery (MCA) territory (Fig. 1B). However, other MRI sequences, including diffusion-weighted imaging, fluid attenuation inversion recovery, T2*-gradient echo imaging, perfusion-weighted MRI, and magnetic resonance angiography, in accordance with the stroke center’s protocol, could not be obtained due to poor cooperation from the patient.
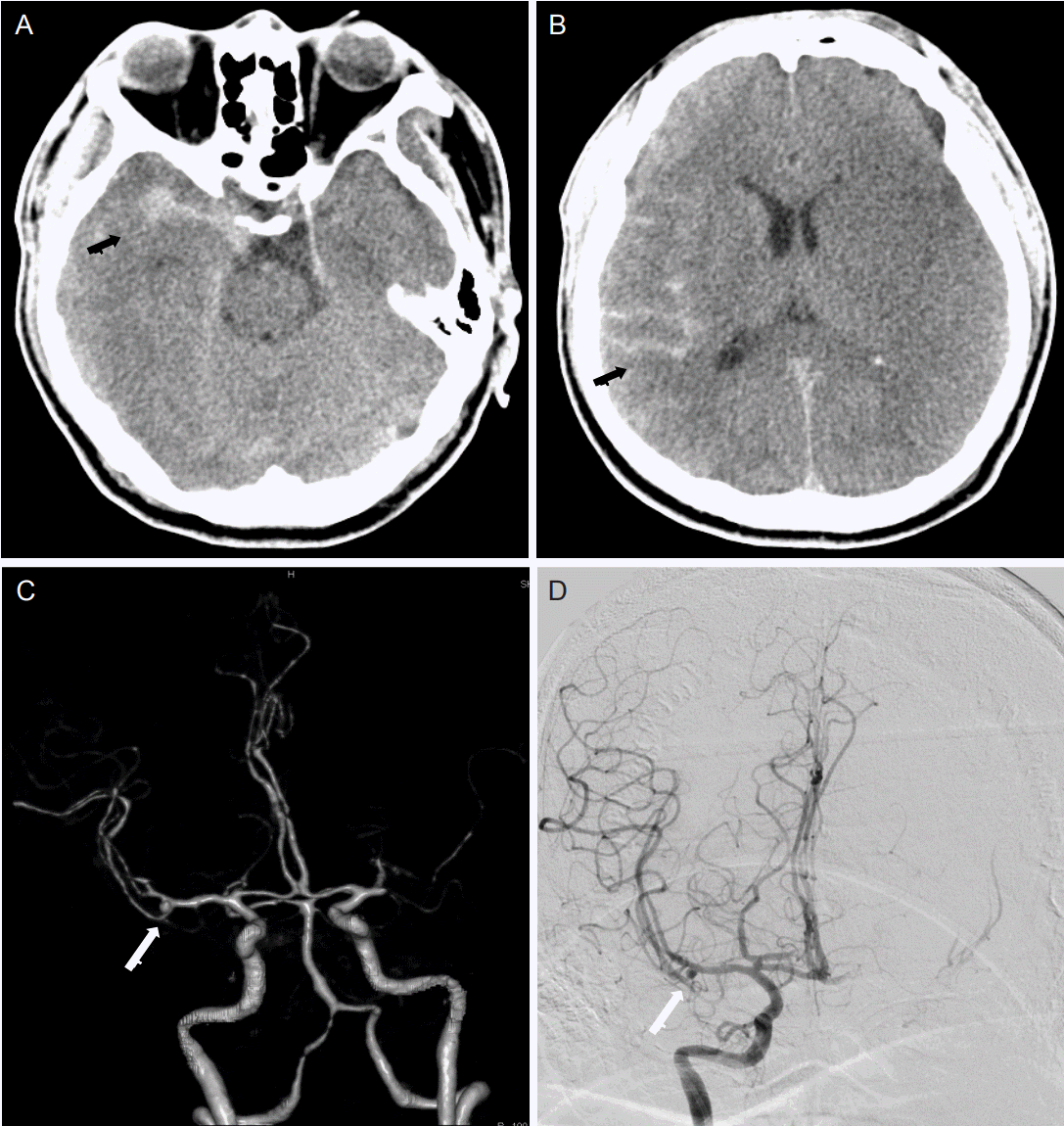
A CT angiography was performed, instead of a magnetic angiography, 2 hours after an infusion of rtPA. During this time, the patient did not exhibit any neurological deterioration. Additionally, the patient’s blood pressure was controlled to < 180/105 mmHg, until the CT scan was performed. Blood pressure was measured twice before the CT angiography, with values of 170/94 mmHg and 157/77 mmHg, and was 169/98 mmHg immediately following the CT angiography. A non-contrast CT, which was performed concurrently with CT angiography, revealed SAH predominantly in the right cerebral convexity and falx (Fig. 2A, B). A CT angiography revealed a saccular aneurysm, 3 mm in size, with a bleb in the right MCA bifurcation and an occlusion of the left MCA M1 portion (Fig. 2C, D). Five hours later, emergent surgical clipping was performed to secure the ruptured aneurysm.
During the operation, a saccular aneurysm with a bleb in the right MCA bifurcation, which was found out in CT angiography, was also observed. After clipping, a thrombectomy of the left MCA was considered, but ultimately was not performed. It was concluded that it was too risky because of the large ischemic area and the presence of SAH. The next morning, brain swelling in the infarcted hemisphere was observed on a follow-up CT scan and decompressive hemicraniectomy was performed 12 hours after the aneurysmal clipping. Targeted temperature management (33°C) was applied for four days due to intracranial hypertension and brain swelling despite hemicraniectomy.
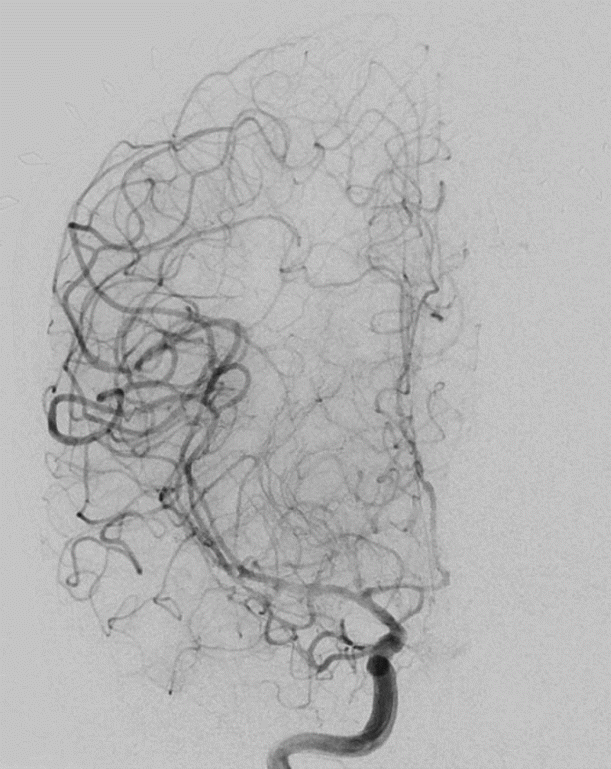
Despite the administration of nimodipine to prevent delayed cerebral infarction, a right MCA territory infarction was detected on a follow-up CT, five days after SAH. However, there were no definitive vasospasms on the conventional angiography (Fig. 3). On discharge five weeks after initial presentation, the patient was alert but had global aphasia and quadriplegia (modified Rankin score 5). His neurological status remained unchanged one year after being discharged.
DISCUSSION
The patient developed SAH in the right cerebral hemisphere―the contralateral side of the infarction―after administration of rtPA. A CT angiography revealed a saccular aneurysm with a bleb in the right MCA bifurcation. Thus, we believe that the unruptured aneurysm ruptured soon after the infusion of rtPA.
To the best of our knowledge, this is the first report to describe a rupture of an aneurysm in the non-infarcted hemisphere soon after an intravenous infusion of rtPA. There have been only three case reports describing aneurysmal SAH that developed after the infusion of rtPA. In the first reported case [8], the patient experienced a thunderclap headache three days before there was a presentation with ischemic stroke. Thus, the patient may have experienced a previous hemorrhage and the re-bleeding was precipitated by rtPA. The second case described a patient with a dissecting aneurysm [9], which did not suggest that an incidental aneurysm ruptured following the infusion of rtPA. In the third case, a previously concealed thrombosed saccular aneurysm was revealed after recanalization [10]. These reports, however, did not convincingly demonstrate that rtPA was the culprit for the ruptured incidental aneurysms. The current case suggests that the infusion of rtPA resulted in the rupture of an intracranial aneurysm in a patient with an acute ischemic stroke.
Several pathophysiological mechanisms may explain the rupture of intracranial aneurysm, after the administration of rtPA. A previous study reported that rtPA can increase turbulence inside an unruptured aneurysm by rapidly dissolving a previous thrombus located at aneurysmal sac. Increased turbulence inside an unruptured aneurysm has been suggested as a possible cause of rupture [12]. Hemorrhagic transformation in the infarcted brain tissue after a rtPA infusion may be related to dysregulated extracellular proteolysis within the neurovascular matrix. In addition, a rtPA infusion can also induce alterations of vascular permeability through the disruption of the integrity of the vascular basal lamina [13].
In conclusion, the current study demonstrates that an incidental intracranial aneurysm may rupture after an intravenous infusion of rtPA in patients who experience acute ischemic stroke. Although recent studies have demonstrated no increase in the risk for rtPA-related intracranial hemorrhage in acute ischemic stroke patients harboring intracranial aneurysm, we suggest that rupture-prone aneurysms, in terms of size and shape, would be considered as a relative contraindication to rtPA. Future studies are needed to investigate whether rtPA is beneficial or harmful in patients who have both acute ischemic stroke and intracranial aneurysms.




 PDF
PDF Citation
Citation Print
Print


 XML Download
XML Download