Abstract
Background
Acute superior mesenteric artery occlusion is a life-threatening disease that often has nonspecific symptoms. However, it should be suspected in those with known risk factors such as atrial fibrillation, cardiac thrombus or cardiac valvular disease.
Acute intestinal ischemia by superior mesenteric artery (SMA) occlusion is a life-threatening disease that requires rapid management. Without early diagnosis and management to restore mesenteric blood flow, SMA occlusion causes extensive bowel necrosis, resulting in a poor prognosis with a high mortality rate up to 80% [1,2].
Since clinical signs and symptoms of acute SMA occlusion are often nonspecific, careful examination and close monitoring is required especially for patients with altered mental status or language function impairment. Risk factors for SMA occlusion include atrial fibrillation, cardiac valvular disease, infective endocarditis, or peripheral vascular disease, which are also known as risk factors of cerebral infarction. A previous study reported a case of SMA occlusion in patient with atrial fibrillation history who was subsequently diagnosed with acute infarction [3]. Earlier autopsy findings of SMA occlusion patients have shown that 2.3% of the patients has concurrent presence of acute ischemic stroke [4], while the incidence of SMA occlusion in the ischemic stroke patients is unknown. Early study reported several cases, in which acute cerebral infarction patients with atrial fibrillation suffered from mesenteric ischemia. However, courses of management were limited due to patient’s early death [5] or refusal of further intervention [6].
Herein, we presented two cases of acute thromboembolic SMA occlusion that developed in patients who were hospitalized for acute cerebral infarction with known atrial fibrillation.
An 85-year-old woman with a history of hypertension, diabetes mellitus and Parkinson disease was referred to our hospital with sudden right-sided weakness. She had not taken any antithrombotic drugs. Neurological examination revealed left gaze preference, right-sided hemianopsia, aphasia and weakness in the right face and extremities. Diffusion weighted image (DWI) indicated high signal intensities in left middle cerebral artery (MCA) territory and magnetic resonance angiography (MRA) showed left proximal MCA occlusion (Fig. 1A, B). Initial electrocardiogram showed atrial fibrillation with rapid ventricular response with a pulse rate of 134/min. Fibrinogen was 486 mg/dL (normal range, 200-400 mg/dL), and B type natriuretic peptide was 560.7 pg/mL (normal range, 0-100 pg/mL). Her transthoracic echocardiogram showed enlarged left atrium with preserved global systolic function with 50% of ejection fraction, and mild global hypokinesia of left ventricle. Twenty-four-hour Holter monitoring confirmed persistent atrial fibrillation with a maximum pulse rate of 213/min. Thyroid function test was in normal range (T3, 0.77 ng/mL; Free T4, 1.2 ng/dL; TSH, 2.99 micro International units [mIU]/mL). Despite no transesophageal echocardiogram and evaluation for cancer marker, considering the absence of evidence for embolism other than atrial fibrillation, , a cardioembolic stroke due to atrial fibrillation was suspected. However, we did not start systemic anticoagulation because of the high risk of hemorrhagic transformation in acute period, especially in large size infarction.
On the 8th hospital day, the patient complained of abdominal pain and diarrhea, and the next day hematemesis and melena occurred. Laboratory data showed anemia, leukocytosis and C-reactive protein elevation (Hemoglobin, 11.9 g/dL; Hematocrit, 34.4%; white blood cell [WBC], 23,210/mm3 ; C-reactive protein, 92.3 mg/dL), and electrocardiogram showed atrial fibrillation with rapid ventricular response with a pulse rate of 160/min. Emergent gastroduodenoscopy revealed reflux esophagitis; and abdominal computed tomography (CT) scan showed diffuse mural thickening in ileum and ascending colon and filling defect in the proximal portion of the SMA main trunk (Fig. 2), which led to the diagnosis of acute SMA occlusion.
Systemic anticoagulation by intravenous heparin was selected as the initial treatment instead of endovascular revascularization. Activated partial thromboplastin time (aPTT) was well controlled (between 60-80 seconds). Abdominal symptoms were gradually improved and follow-up abdominal CT at 12 days after anticoagulation showed decreased thrombus in proximal SMA. However, brain CT on the same day revealed hemorrhagic transformation of infarcted tissue (Fig. 1C). We continued systemic anticoagulation and switched heparin to apixaban 2.5 mg twice-daily, considering the patients’ age, body weight (53.6 kg), renal function (creatinine, 0.73 mg/dL), brain hemorrhagic transformation and nonvalvular atrial fibrillation.
A 75-year-old-man with a history of hypertension, persistent atrial fibrillation, active tuberculosis, and hypothyroidism was referred to our hospital with sudden left-sided hemianopsia and sensory extinction. He had been taking warfarin for 7 years. DWI of the brain magnetic resonance image (MRI) showed high signal intensities in territory of inferior division of right MCA, and MRA showed severe stenosis of right distal MCA. Initial electrocardiogram showed atrial fibrillation with slow ventricular response, and pulse rate was 46/min. Transthoracic echocardiogram showed enlarged left ventricle with 64% of ejection fraction, and a moderate atrial regurgitation due to degenerative change of atrial valve. Transesophageal echocardiogram and cancer marker evaluation was not done. His heart CT showed no aortic arch plaque which may have caused embolism. Thyroid function test (T3, 0.26 ng/mL; Free T4, 0.73 ng/dL; TSH, 3.38 uIU/mL) indicated no thyroid disease. Fibrinogen was 388 mg/dL, and D-dimer was 1,004 ng/mL (normal range, 0-243 ng/mL). Based on these findings, we suspected cardioembolic cerebral infarction caused by the atrial fibrillation. International normalization ratio (INR) was 1.74, so we dosed up warfarin to reach effective anticoagulation.
During admission, we had difficulty in control of anticoagulation. In the morning on the 9th hospital day, we decided to further increase the dose of warfarin after determining INR of 1.53, which was decreased compared to 2.66 on the 8th hospital day. In the evening on the same day, he complained of acute and persistent periumbilical pain without abdominal distension. Bowel sound was decreased, but nausea or vomiting were absent. Laboratory data showed leukocytosis and elevated C-reactive protein (WBC, 15,080/mm3; Hemoglobin, 12.0 g/dL; C-reactive protein, 41.9 mg/dL), D-dimer was 3,015 ng/mL. Electrocardiogram showed atrial fibrillation with rapid ventricular response with a pulse rate of 135/min. Abdominal CT scan revealed decreased enhancement of the pelvic small bowel loops, without definite evidence of bowl infarction and suspicious intraluminal filling defect at the SMA (Fig. 3A). Angiography showed a thromboembolic occlusion at proximal SMA and catheter-directed thrombolysis was conducted using 100,000 International units (IU) of urokinase (Fig. 3B). On the 10th hospital day, surgical exploration was decided due to persistent abdominal pain and decreased bowl movement. Ischemic change was observed in a small bowel and in the part of transverse colon. Damaged bowel was resected and surgical thrombectomy was done. Follow-up abdominal CT scan on the 1st post-operative day showed persistent filling defect in the proximal SMA, and gas in distal arterial SMA branches and mesenteric veins, which indicated unresolved thrombotic occlusion in the proximal SMA (Fig. 3C). The 2nd-look operation was planned but patient’s family requested no further management. On the 9th post-operative day, patient expired.
SMA occlusion results in high mortality, with earlier studies showing rates up to 80% [1,2]. Early diagnosis and treatment before the sign of bowel necrosis is crucial, since mortality increases progressively with time [7,8]. Frequently, acute mesenteric ischemia remains unrecognized initially due to its non-specific symptoms [9]. Once diagnosed, treatment approach should be decided to restore intestinal perfusion as quickly as possible [10]. Although it is controversial, recent studies show that endovascular approach such as catheter-directed thrombolysis, balloon angioplasty and stent may be as effective as surgical treatment including surgical thrombectomy and resection of necrotic bowl in acute SMA occlusion patients [11,12].
Causes of SMA occlusion include embolism from cardiogenic sources (atrial fibrillation, cardiac thrombus or valvular disease), which are also reported to be associated with high risk for cerebral infarction. Although several cases were reported previously, there were limitations in management due to patient’s early death or refusal of treatment.
In the case 1, the patient was diagnosed with SMA occlusion during the time she was not administered with anticoagulation because of a risk of cerebral hemorrhagic transformation. The patient in the case 2 suffered from SMA occlusion when under therapeutic anticoagulation. The timing of anticoagulation is another problem in patients with simultaneous cerebral infarction and SMA occlusion. However, it may indicate that effective anticoagulation has benefits in prevention and treatment of SMA occlusion in cerebral infarction patients with atrial fibrillation, and that we should carefully observe nonspecific symptoms of SMA occlusion especially when anticoagulation of cerebral infarction patients with atrial fibrillation is uncontrolled.
In addition, atherosclerosis of aorta and its branches may play a role in SMA occlusion in atrial fibrillation patients. In the case 1, abdominal CT scan revealed multifocal calcified lesions in the abdominal aorta, and in the case 2, severe stenoses and chronic occlusions of ileal branch arteries were shown in angiography and the lower ileum was perfused by collateral arteries. Considering non-specific symptoms, and significance of early detection and treatment in acute SMA occlusion, early diagnosis and management plan for SMA occlusion and effective anticoagulation are important. Also, we recommend close observation for gastro-intestinal symptoms and signs in cerebral infarction patients with atrial fibrillation.
REFERENCES
1. Mamode N, Pickford I, Leiberman P. Failure to improve outcome in acute mesenteric ischaemia: seven-year review. Eur J Surg. 1999; 165:203–8.
2. Vokurka J, Olejnik J, Jedlicka V, Vesely M, Ciernik J, Paseka T. Acute mesenteric ischemia. Hepatogastroenterology. 2007; 55:1349–52.
3. Takeyama K, Miura M, Nakayama K, Suzuki T. A Case of Superior Mesenteric Artery Occlusion Caused by Delayed Administration of Anticoagulants in a Patient with Nonvalvular Atrial Fibrillation. Tokai J Exp Clin Med. 2016; 41:4–7.
4. Acosta S, Ogren M, Sternby NH, Bergqvist D, Bjorck M. Clinical implications for the management of acute thromboembolic occlusion of the superior mesenteric artery: autopsy findings in 213 patients. Ann Surg. 2005; 241:516–22.
5. Oguro H, Yamaguchi S. Acute mesenteric ischemia after recurrent embolic stroke. Int J Gastroenerol Disord Ther. 2015; 2:117.

6. Lee MK, Lee DW, Cho KH, Nam HS, Heo JH, Kim YD. Superior mesenteric artery occlusion in acute cardioembolic stroke. J Korean Neurol Assoc. 2009; 27:299–300.
7. Bingol H, Zeybek N, Cingöz F, Yilmaz AT, Tatar H, Şen D. Surgical therapy for acute superior mesenteric artery embolism. Am J Surg. 2004; 188:68–70.

8. van Petersen AS, Kolkman JJ, Meerwaldt R, Huisman AB, van der Palen J, Zeebregts CJ, et al. Mesenteric stenosis, collaterals, and compensatory blood flow. J Vasc Surg. 2014; 60:111–9.

9. Park WM, Gloviczki P, Cherry KJ Jr, Hallett JW Jr, Bower TC, Panneton JM, et al. Contemporary management of acute mesenteric ischemia: Factors associated with survival. J Vasc Surg. 2002; 35:445–52.

10. Anderson JL, Halperin JL, Albert NM, Bozkurt B, Brindis RG, Curtis LH, et al. Management of patients with peripheral artery disease (compilation of 2005 and 2011 ACCF/AHA guideline recommendations): a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. Circulation. 2013; 127:1425–43.
Figure 1.
(A) Diffusion-weighted MRI shows diffusion restriction in the left middle cerebral artery territory. (B) MR angiography shows occlusion (arrow) of left proximal middle cerebral artery. (C) Brain non-contrast CT shows hemorrhagic transformation in the left middle cerebral artery territory. (D) DWI shows diffusion restriction in the right MCA territory. (E) MR angiography shows occlusion (arrow) of right distal MCA. MRI, magnetic resonance imaging; CT, computed tomography; DWI, diffusion weighted image; MCA, middle cerebral artery.
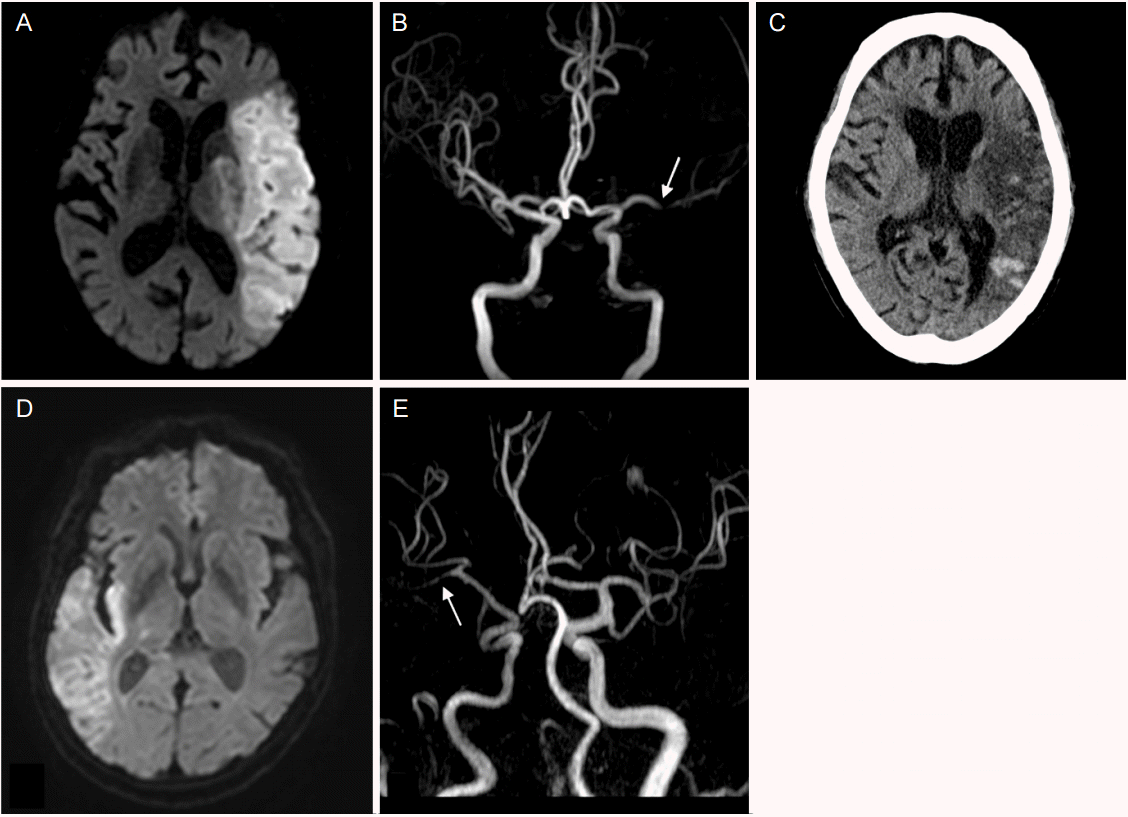
Figure 2.
(A) Abdominal computed tomography scan shows filling defect (1.4 cm, arrow) in length in proximal superior mesenteric artery. (B) Follow up CT 12 days after anticoagulation shows decreased thrombus (0.7 cm, arrow) with partial recanalization. CT, computed tomography.
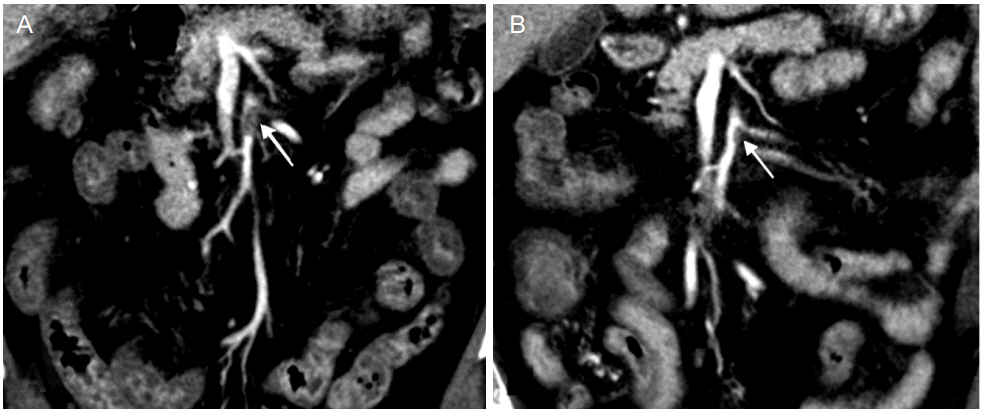
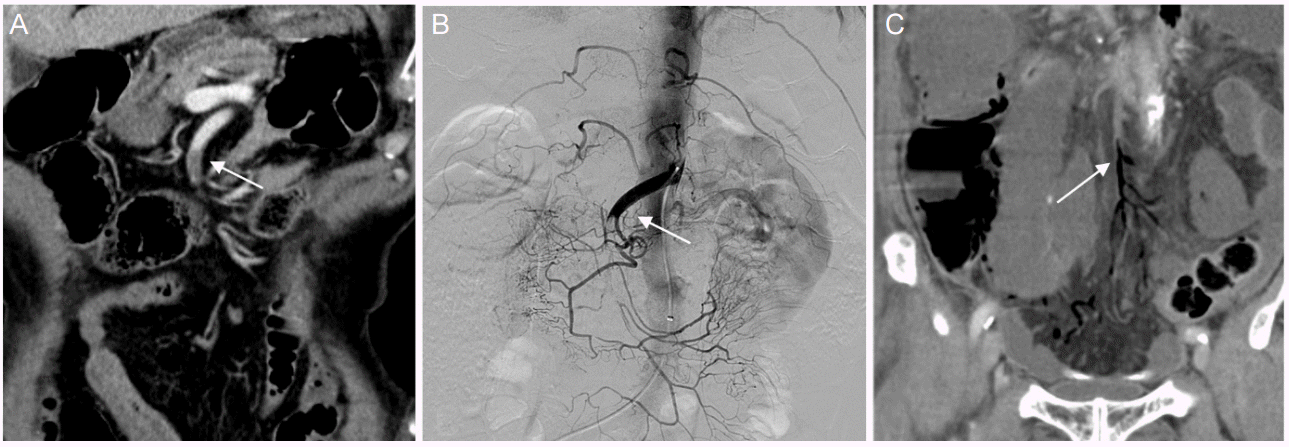
Figure 3.
(A) Abdominal computed tomography scan shows filling defect (arrow) in proximal superior mesenteric artery. (B) Superior mesenteric angiography shows proximal SMA occlusion (arrow). (C) Follow up CT after catheter-directed thrombolysis shows gas (arrow) in SMA distal arterial branch and mesenteric vein. CT, computed tomography; SMA, superior mesenteric artery.




 PDF
PDF Citation
Citation Print
Print


 XML Download
XML Download