Abstract
Background:
Total knee replacement is often accompanied by severe post-operative pain. Oxycodone has sufficient analgesic effects and somewhat greater, but tolerable side effects compared to fentanyl. However, most studies on the topic evaluate visceral pain relief. In this study, we determine the effectiveness of oxycodone for somatic pain and evaluate the incidence of side effects.
Methods:
Sixty-nine patients were involved in a randomized control trial. Analgesic agents were administered to two experimental groups at a post anesthetic care unit (PACU) 15 min after PACU admission: a 50 μg fentanyl group (n = 40) and a 4 mg oxycodone group (n = 29), both with severe pain (numeric rating scale, NRS > 5). Changes in NRS at the PACU were measured. Additional analgesic agents were administered at 0–6, 6–12, 12–24, and 24–48 h after surgery.
Go to : 
Post-operative pain management is an important factor in improving quality of life during hospital stays for total knee replacement (TKR) patients [1]. Opioids are known to be effective for post-operative pain control. Morphine and fentanyl are commonly used opioids, but there is a lack of research on oxycodone’s potential as a suitable replacement for fentanyl [2]. Oxycodone is a strong, pure, and semisynthetic opioid that acts as an agonist of the central, peripheral, and autonomous nervous systems. There are several studies of its efficacy and safety in treating both acute and chronic moderate-to-severe post-operative or cancer pain without ceiling effects [3-6]. Oxycodone can be administered through many routes: intravenous [7,8], intramuscular [9], rectal [10], subcutaneous [11], intranasal [8], epidural [12], and oral [9].
There are several studies suggesting that oxycodone has a comparable analgesic effect and somewhat greater, but tolerable side effects compared to fentanyl as an adjunctive drug [13-15]. However, most of the studies evaluated visceral pain relief.
In this study, we investigated whether oxycodone is an effective substitute for fentanyl for early post-operative pain management in orthopedic surgery. We also compared possible side effects in both groups.
Go to : 
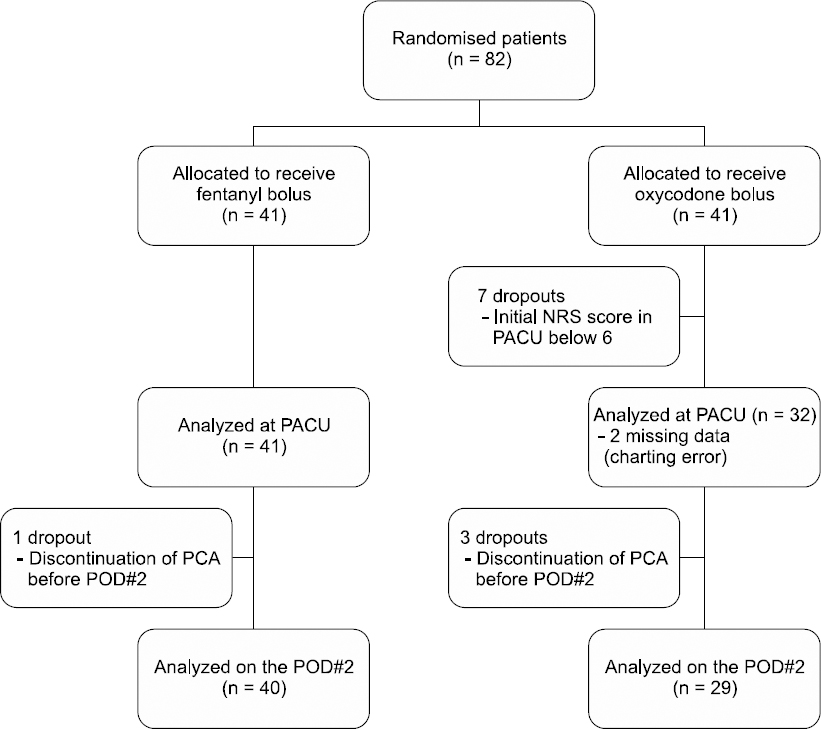
A prospective, randomized controlled trial and single-blind study was conducted, and patients were given informed consent before surgery. Patients with a history of hepatitis and renal failure, habitual drug use, mental illnesses, and side effects for previous opioid history were excluded from the study. Eighty-two patients were scheduled for total knee replacement. Patients were randomly divided into two groups using Excel (Microsoft Corp., Korea): a group receiving 50 μg fentanyl (Group F, n = 40) and a group receiving 4 mg oxycodone (Group O, n = 29). Diagrams of each group are presented in Fig. 1. After screening, a total of 69 patients designated as American Society of Anesthesiologists physical status class I to II were included in this study. We used the numerating scale (NRS) calculation method (NRS; 0 = no pain, and 10 = most severe pain) for measuring pain severity.
A standardized, general anesthetic technique was used for each patient. In the operating room, all patients were continuously monitored with electrocardiography, pulse oximetry, bispectral index, blood pressure, and capnography. We administrated glycopyrrolate 0.2 mg and palonosetron 0.075 mg for premedication. Anesthesia was induced with 1–2 mg/kg propofol and 0.8 mg/kg rocuronium. Desflurane (5–7 vol%) was used with a mixture of nitrous oxide (50%) and oxygen (50%) for anesthesia maintenance. Remifentanil was also infused at 1.5–5.0 μg/min. Fifteen minutes before the end of surgery, 50 μg of fentanyl bolus was administered. Following full skin closure, desflurane and remifentanil were discontinued. The reversal agent was mixture of 0.4 mg glycopyrrolate and 15 mg pyridostigmine. Tracheal extubation was performed after self-respiration was recovered and the patient’s eyes opened. All patients received intravenous patient-controlled analgesia (IV PCA) with 15 μg/kg fentanyl for 2 days (0.31 μg/kg/h). Additional intravenous fentanyl bolus was used instead of pushing a PCA bolus button in the postoperative period.
After surgery was complete, patients were transferred to the post anesthetic care unit (PACU), and observed for at least 30 min before being transferred to the general ward. In a double-blind manner, the analgesic agent (either 50 μg fentanyl or 4 mg oxycodone) was administered depending on whether the patient’s NRS exceeded 5, and we assessed the NRS 15 min later. Additionally, the patient’s post-operative nausea and vomiting (PONV), dizziness, pruritus (itching), headache, respiratory depression, and sedation grade (awake and alert, occasionally drowsy, easily aroused, asleep) in the PACU were all observed.
In the general ward, if patients requested more pain relief, 50 μg of fentanyl were administered. The time of the first administration of analgesia was monitored, as was total fentanyl consumption via intravenous bolus and PCA during the periods of 0–6, 6–12, 12–24, and 24–48 h after surgery.
The primary endpoint was the difference in fentanyl consumption during the acute postoperative period (0–6 h). Based on a pilot study, the difference and standard deviation between the two groups were expected to be 25 and 29 μg, respectively. The sample size was calculated using SigmaPlot 13.0 (Systat Software Inc., USA). The minimum sample size required in each group with a significance criterion (α) of 0.05 and a statistical power of 0.8 was 23. All statistical analyses were carried out using SPSS software (version 21.0, IBM Corp., USA). Mann-Whitney U tests or unpaired t-tests were performed to compare continuous variables between the two groups. Discrete variables, side effects, and the proportion of patients who required additional opioids were analyzed using the Chi-square test. P value less than 0.050 was considered statistically significant. Data are presented as the mean ± SD deviation unless otherwise noted.
Go to : 
The study involved 69 patients. Demographic and clinical characteristics of the patients organized according to group are shown in Table 1. There were no statistically significant differences between the two groups in patient age, weight, height, duration of surgery, and total remifentanil consumption (P > 0.050)
Demographic Data and Anesthesia Characteristics
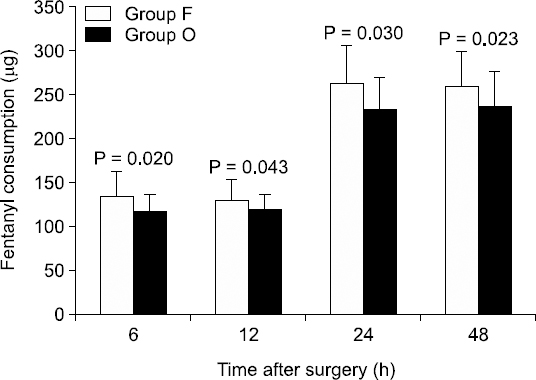
Total fentanyl consumption during the periods of 0–6, 6–12, 12–24, and 24–48 h after surgery was calculated. Significantly less fentanyl was consumed in Group O compared to Group F (P < 0.050) during each of the time periods (Fig. 2).
The proportion of patients who required additional opioids was also counted during the periods of 0–6, 6–12, 12–24, and 24–48 h after surgery (Table 2). A significantly lower proportion was observed in Group O than in Group F during 0–6 and 12–24 h.
Pain scores before and after administration of analgesics (Table 3) did not differ significantly between the two groups at PACU (P > 0.050).
Incidence rates of adverse events were recorded throughout the 48 h of the experiment (Table 4). Twenty patients experienced nausea/vomiting (Group F, n = 11; Group O, n = 9) and all of them were successfully relieved via antiemetic agents (palonosetron, and metoclopramide). None of them had dizziness/headache in the PACU, and one patient in Group F experienced pruritis. Two patients in Group O occasionally fell asleep after analgesic administration but were easily aroused. However, there was no significant difference between two groups (P > 0.050).
Go to : 
Opioids are widely used for post-operative pain management; these include morphine, the fentanyl series, oxycodone, pethidine, etc. Oxycodone is a semisynthetic opioid derived from thebaine, and acts as an agonist on mu, kappa, and delta receptors in the central nervous system. The plasma half-life of oxycodone is 3–5 h, with stable plasma concentration levels reached within 24 h [16].
Most studies on the pain control abilities of oxycodone involve PCA infusion in the ward [14,15,17]; there are few studies in the PACU. In addition, oxycodone is known to be effective for visceral pain, thus studies are focused solely on visceral pain [13]. Therefore, we studied whether oxycodone is an effective analgesic agent for somatic pain as well.
TKR is often accompanied by severe post-operative pain. Post-operative pain is the most common reason for delayed rehabilitation, prolonged hospital stays, and chronic pain after a TKR [17]. In the acute postoperative phase, most patients reported severe pain (NRS 6–7) in this study despite PCA infusions. Severe acute pain is likely to lead to chronic pain [13], so in the PACU, adequate and prophylactic pain management is necessary. In this study, the oxycodone group showed lower fentanyl consumption not only during the 0–6 h postoperative period but also the 6–48 h period. Therefore, we conclude that oxycodone is a suitable analgesic for management of acute postoperative pain.
While IV oxycodone is known to be equipotent to morphine, it is reported that the potency ratio of fentanyl to morphine is 1:80–100 [10]. In addition, Herrick et al. [18] compared IV PCA (fentanyl) with IV PCA (morphine) in TKR patients and concluded that the fentanyl group needed more PCA bolus but reported the same VAS score in fentanyl to morphine ratio 1:100. Hwang et al. [2] have suggested that the potency ratio of fentanyl to oxycodone is approximately 1:75 and that oxycodone might be more potent than morphine (4:3). Therefore, we administered fentanyl at 50 μg for fentanyl group and oxycodone at 4 mg for oxycodone group.
Analgesic effects were measured using NRS in the PACU. It appears that oxycodone has a longer analgesic effect than fentanyl, because the oxycodone group reported lower total fentanyl consumption and a lower proportion of patients who demanded additional opioids. Therefore, we conclude that oxycodone is more effective than fentanyl in the acute phase of post-operative pain control. In previous studies, fentanyl has been shown to provide almost immediate analgesic effects when administered intravenously, whereas oxycodone takes effect 10–15 min after administration [7,10]. Our study showed that the change in NRS between group F and group O was not different at 15 min.
Common side effects of oxycodone are constipation (around 25–30% of patients), nausea (25%), drowsiness (25%), dizziness (15%), vomiting (10–15%), and pruritus (10–15%) [16]. In this study, the higher incidence of PONV may be due to bolus opioids that were complementary to PCA. In addition, the occurrence of nausea after surgery may be due to other factors such as the impact of the effects of inhaled anesthetics and surgical pain [19]. Most nausea occurred within 24 h and showed a tendency to decrease over time in both groups. No patients reported dizziness or headache, while two people fell asleep but were easily aroused. However, there were no significant differences in side effects between two groups (P > 0.050).
There are several limitations in this study. The number of patients in the oxycodone group is lower than that in the fentanyl group. Furthermore, there is more missing data in group O than in group F. More balanced grouping will be important in future studies. As mentioned previously, we administered 4 mg of oxycodone. However, Park et al. [14] suggested that a potency ratio of fentanyl to oxycodone of 1:60 is sufficient for an analgesic effect, while minimizing side effects. We should consider the overestimation of potency ratios because our patients used IV PCA in combination with fentanyl. Therefore, additional studies using various potency ratios will be needed. Although there is no need to reduce dose with age [16], we report that most patients were elderly and female. There is a need to conduct research with a broader patient population.
In conclusion, oxycodone shows better analgesic effects than fentanyl for acute somatic pain during the post-operative period. Side effects are not significantly greater than fentanyl. Thus, oxycodone is a reasonable choice as an alternative to fentanyl.
Go to : 
REFERENCES
1. Seo DK, Lee CJ, Kim JS. A comparison of oxycodone and fentanyl in the management of early postoperative pain and for patient-controlled analgesia after total abdominal hysterectomy. Anesth Pain Med. 2016; 11:176–81. DOI: 10.17085/apm.2016.11.2.176.

2. Hwang BY, Kwon JY, Kim E, Lee DW, Kim TK, Kim HK. Oxycodone vs. fentanyl patient-controlled analgesia after laparoscopic cholecystectomy. Int J Med Sci. 2014; 11:658–62. DOI: 10.7150/ijms.8331. PMID: 24843313. PMCID: PMC4025163.

3. Kalso E, Pöyhiä R, Onnela P, Linko K, Tigerstedt I, Tammisto T. Intravenous morphine and oxycodone for pain after abdominal surgery. Acta Anaesthesiol Scand. 1991; 35:642–6. DOI: 10.1111/j.1399-6576.1991.tb03364.x. PMID: 1785245.

4. Nuutinen LS, Wuolijoki E, Pentikäinen IT. Diclofenac and oxycodone in treatment of postoperative pain: a double-blind trial. Acta Anaesthesiol Scand. 1986; 30:620–4. DOI: 10.1111/j.1399-6576.1986.tb02487.x. PMID: 3544648.
5. Tigerstedt I, Leander P, Tammisto T. Postoperative analgesics for superficial surgery. Comparison of four analgesics. Acta Anaesthesiol Scand. 1981; 25:543–7. DOI: 10.1111/j.1399-6576.1981.tb01702.x. PMID: 6810642.
6. Tammisto T, Tigerstedt I, Korttila K. Comparison of lysine acetylsalicylate and oxycodone in postoperative pain following upper abdominal surgery. Ann Chir Gynaecol. 1980; 69:287–92. PMID: 6782930.
7. Pöyhiä R, Olkkola KT, Seppälä T, Kalso E. The pharmacokinetics of oxycodone after intravenous injection in adults. Br J Clin Pharmacol. 1991; 32:516–8. DOI: 10.1111/j.1365-2125.1991.tb03942.x. PMID: 1958450. PMCID: PMC1368617.
8. Takala A, Kaasalainen V, Seppälä T, Kalso E, Olkkola KT. Pharmacokinetic comparison of intravenous and intranasal administration of oxycodone. Acta Anaesthesiol Scand. 1997; 41:309–12. DOI: 10.1111/j.1399-6576.1997.tb04684.x. PMID: 9062618.

9. Pöyhiä R, Seppälä T, Olkkola KT, Kalso E. The pharmacokinetics and metabolism of oxycodone after intramuscular and oral administration to healthy subjects. Br J Clin Pharmacol. 1992; 33:617–21. DOI: 10.1111/j.1365-2125.1992.tb04090.x. PMID: 1389934. PMCID: PMC1381353.
10. Leow KP, Cramond T, Smith MT. Pharmacokinetics and pharmacodynamics of oxycodone when given intravenously and rectally to adult patients with cancer pain. Anesth Analg. 1995; 80:296–302. DOI: 10.1097/00000539-199502000-00016. PMID: 7818116.

11. Maddocks I, Somogyi A, Abbott F, Hayball P, Parker D. Attenuation of morphine-induced delirium in palliative care by substitution with infusion of oxycodone. J Pain Symptom Manage. 1996; 12:182–9. DOI: 10.1016/0885-3924(96)00050-4.

12. Backlund M, Lindgren L, Kajimoto Y, Rosenberg PH. Comparison of epidural morphine and oxycodone for pain after abdominal surgery. J Clin Anesth. 1997; 9:30–5. DOI: 10.1016/S0952-8180(96)00212-7.

13. Koch S, Ahlburg P, Spangsberg N, Brock B, Tønnesen E, Nikolajsen L. Oxycodone vs. fentanyl in the treatment of early post-operative pain after laparoscopic cholecystectomy: a randomised double-blind study. Acta Anaesthesiol Scand. 2008; 52:845–50. DOI: 10.1111/j.1399-6576.2008.01643.x. PMID: 18477082.
14. Park JH, Lee C, Shin Y, An JH, Ban JS, Lee JH. Comparison of oxycodone and fentanyl for postoperative patient-controlled analgesia after laparoscopic gynecological surgery. Korean J Anesthesiol. 2015; 68:153–8. DOI: 10.4097/kjae.2015.68.2.153. PMID: 25844134. PMCID: PMC4384403.

15. Silvasti M, Rosenberg P, Seppälä T, Svartling N, Pitkänen M. Comparison of analgesic efficacy of oxycodone and morphine in postoperative intravenous patient-controlled analgesia. Acta Anaesthesiol Scand. 1998; 42:576–80. DOI: 10.1111/j.1399-6576.1998.tb05169.x. PMID: 9605375.

16. Ordóñez Gallego A, González Barón M, Espinosa Arranz E. Oxycodone: a pharmacological and clinical review. Clin Transl Oncol. 2007; 9:298–307. DOI: 10.1007/s12094-007-0057-9.

17. Bisgaard T, Klarskov B, Rosenberg J, Kehlet H. Characteristics and prediction of early pain after laparoscopic cholecystectomy. Pain. 2001; 90:261–9. DOI: 10.1016/S0304-3959(00)00406-1.

18. Herrick IA, Ganapathy S, Komar W, Kirkby J, Moote CA, Dobkowski W, et al. Postoperative cognitive impairment in the elderly. Choice of patient-controlled analgesia opioid. Anaesthesia. 1996; 51:356–60. DOI: 10.1111/j.1365-2044.1996.tb07748.x. PMID: 8686825.

19. Watcha MF, White PF. Postoperative nausea and vomiting. Its etiology, treatment, and prevention. Anesthesiology. 1992; 77:162–84. DOI: 10.1097/00000542-199207000-00023.

Go to : 




 PDF
PDF Citation
Citation Print
Print


 XML Download
XML Download