CASE REPORT
A 57-year-old man with left rotator cuff tear was admitted for arthroscopic repair under general anesthesia. The patient’s height and weight were 169 cm and 84.7 kg, respectively. He was classified into the American Society of Anesthesiologists physical status I group.
The patient was not premedicated. Monitoring included non-invasive blood pressure, electrocardiography (lead II, aVL), pulse oximetry (SpO
2), bispectral index (BIS), and end-tidal CO
2 concentration (EtCO
2) measurements. Initial blood pressure was 150/90 mmHg. Heart rate was 65 beats/min, and SpO
2 was 99%. Then, BPB was performed with the interscalene access using ultrasonography for the control of postoperative pain. The block was achieved with administration of 0.75% ropivacaine 15 ml. Anesthesia was induced by administration of 100% O
2 via a mask. Fentanyl 50 μg, 1% lidocaine 40 mg, propofol 150 mg, and rocuronium 50 mg were administered intravenously. Laryngoscopy was performed using a Macintosh blade (No. 3). The trachea was intubated using an 8.0 mm cuffed endotracheal tube. The intubation was easily performed on a single attempt on a grade I view of the glottic opening. The cuff of the tube was inflated to a cuff pressure of less 20 cmH
2O. Anesthesia was maintained using 2 L/min O
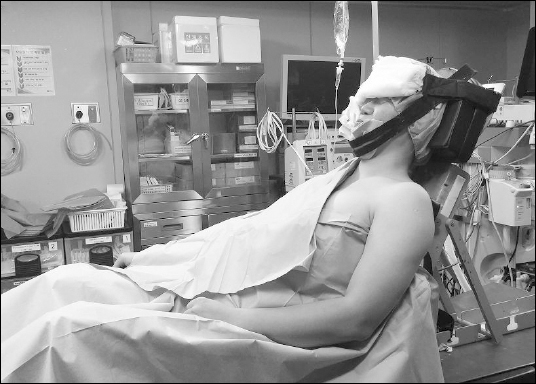
2, 2 L/min nitrous oxide, and 3–4 vol% desflurane. The patient was placed in the beach chair position with the knee in flexion. The patient’s head and neck were secured in the neutral position with two straps, which were positioned on the chin and forehead (
Fig. 1). When the surgical drapes were opened after the operation, the patient’s head was found to be in excessive lateral extension to the right, about 30–40°. Glycopyrrolate 0.4 mg and pyridostigmine 10 mg were administered for the reversal of muscle relaxation. Tracheobronchial suction was performed with 14 Fr. suction catheter after self respiration was restored and the patient woke up from anesthesia. Then, the patient was extubated without any trauma. The operation time was 90 minutes and the anesthesia time was 140 minutes.
Fig. 1
Beach chair position for arthroscopic shoulder surgery.
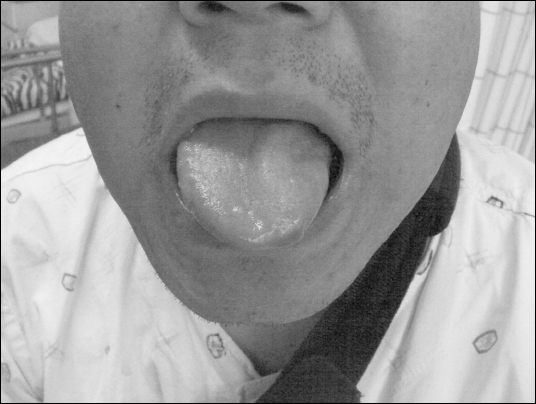
Complications were not detected during recovery in the postanesthetic care unit. But, the patient complained of slurred speech after being transferred to the ward. Physical examination revealed asymmetrical facial features with dysarthria and tongue deviation to the left (
Fig. 2). There were no abnormal findings of the vocal cord and uvula on laryngoscopic examination at the otolaryngology clinic. Diffusion MRI revealed normal findings without ischemia, tumor, or hemorrhage. Neurologic consultation confirmed that the left hypoglossal nerve was damaged after the function of cranial nerves was examined; gag reflex, sensation of the mouth and tongue, tongue protrusion or atrophy, etc. Vitamin B (1, 6, 12) 25 mg once a day and ginkgo biloba 40 mg three times a day were given for 2 weeks, but steroids were not given.
Fig. 2
Left hypoglossal nerve injury causes an inability to stick the right part of the tongue out straight, and it deviates towards the left side due to nerve damage.
Asymmetry of the face improved after postoperative 24 hours, but, the other symptoms were still present. Other neurologic symptoms subsided after postoperative 15 days and the patient was discharged without complications.
DISCUSSION
Peripheral nerve injury after shoulder surgery may often be caused by surgical traction, with an incidence of 4%. Cranial nerve injury is rare after surgery that has not been performed on the head and neck [
3]. Especially, the incidence of isolated hypoglossal nerve injury is very low. There were 4 cases of isolated hypoglossal nerve injury after shoulder surgery in the beach chair position [
2,
4,
5]. Shah et al. [
6] reported that there were 46 patients with isolated hypoglossal nerve palsy after airway management for general anesthesia in the literature from 1926 to 2013.
Hypoglossal nerve injury is associated with use of laryngeal mask airway and endotracheal intubation, malignancy, trauma, stroke, multiple sclerosis, Guillain-Barre syndrome, infection, and even hysteria [
7-
9]
The sites that may be affected by hypoglossal nerve palsy are the hypoglossal nucleus of the medulla oblongata, fibers in the medulla oblongata, cistern, and hypoglossal canal, as well as along the extracranial portion. Lesions of the intracranial portion lead to multiple neurapraxias or the long tract signs (Babinski’s sign, Hoffman’s sign, ankle clonus, etc.) [
2]. In our case, there were no long tract signs and other lesions on MRI, and only isolated hypoglossal neurapraxia was seen. Suspected mechanisms of hypoglossal nerve injury in the extracranial portion are related to anatomic course of the hypoglossal nerve. The hypoglossal nerve is a pure motor nerve that innervates the tongue. It exits the skull through the hypoglossal canal in the occipital bone and travels a course through the neck, ultimately reaching the mylohyoid and hyoglossus muscles, placing it in close proximity to airway instrumentation devices [
2]. Hypoglossal nerve injury is related to airway management such as stretching of the nerve against the greater horn of the hyoid bone by a laryngeal mask or orotracheal tube or compression of the nerve by the posterior part of the laryngoscope, distension of the nerve during intubation with application of cricoid pressure, and inadvertent extubation of the tracheal tube with its cuff still inflated [
10]. But, we quickly performed laryngoscopy on a single attempt, passing the endotracheal tube without any trauma. The depth of the endotracheal tube was 23 cm at the lips, which was sufficient to inflate the cuff below the vocal cords and place the cuff well below the cornu of the hyoid bone. The patient did not have any symptoms of laryngeal injury that include severe throat pain or laryngeal edema. There was no trauma or abnormal finding in the larynx on otolaryngology consultation. But, hypoglossal nerve injury associated with laryngoscopy and intubation might occur under multifactorial conditions [
6]. Therefore, we could not exclude the possibility of injury due to laryngoscopy and intubation.
The hypoglossal nerve exits the skull, then descends between the internal carotid artery and internal jugular vein, and passes inferiorly to the angle of the mandible. Therefore, direct compression of the hypoglossal nerve beneath the angle of the mandible, internal carotid artery dissection, and central venous catheterization may be the possible etiologies of hypoglossal nerve palsy [
10]. In the beach chair position, the strap used to secure the head in the neutral position might compress the hypoglossal nerve directly. Sometimes the surgical assistant’s elbow can compress the mandible angle during surgery [
10]. As the surgical drapes were removed after the operation, the patient’s head was found to be in excessive lateral extension. The manipulation during shoulder surgery may have contributed to hyperextension of the neck leading to hypoglossal nerve injury. In addition, the muscle relaxant used in general anesthesia could have reduced the muscle tone and increase the risk of head movement [
3]. Therefore, the hypoglossal nerve may have been injured by a prolonged stretching mechanism caused by marked lateral extension of the neck and direct compression by the strap at the angle of mandible in our patient.
Cogan et al. [
1] have described that during surgery, any change in position which modifies the angle of the trunk in relation to the headrest could cause nerve compression under the angle of the jaw. Positional injury can occur due to IX, X, XI, XII cranial nerve compression and pulling, or “rack” type effects caused by various anatomical structures. Therefore, if some of the cranial nerves are injured, neurologic function of other nerves should be checked.
Johnson and Moore [
11] reported a case of cranial nerve X and XII paralysis after an interscalene BPB for shoulder surgery. Also, Bharati et al. [
12] experienced a case of paralysis of hypoglossal nerve following interscalene BPB. They performed interscalene BPB blindly and used higher dose (50 ml and 25 ml) of the local anesthetic. The injected area was compressed by dry gauge pieces with the idea to spread the drug along the brachial plexus. Spread of the local anesthetic could block the cranial nerve X and/or XII as well as the brachial plexus. In our case, BPB was performed using ultrasonography and we administered a lower dosage (15 ml). The use of ultrasonography or a nerve stimulator has increased the success rate of nerve block and reduced the incidence of complications.
Gautier et al. [
13] reported that the minimum effective volume of 0.75% ropivacaine in ultrasound-guided interscalene brachial plexus was 5 ml (1.7 ml/trunk). But, because we used higher dosage, unwanted spread of ropivacaine towards the central nervous system might have occurred. In addition, we performed general anesthesia immediately after BPB, and we could not confirm the extent of the nerve block. Therefore, we cannot exclude the possibility that hypoglossal nerve palsy might have been caused by BPB.
Treatment of nerve injury is largely supportive. There is no beneficial protocol for cranial nerve injuries in the literature. But, corticosteroids may reduce secondary injury by reducing tissue edema and inflammation. In addition to steroid therapy, anti-inflammatory drugs or vitamin B is beneficial. Sometimes, speech and language therapy or psychiatric therapy may be necessary [
7]. The symptoms of peripheral damage to cranial nerves develop within 48 hours postoperatively and resolve spontaneously within 4–6 months [
7].
To prevent this complication, the head position should be checked frequently during shoulder surgery. We must pay attention to the strap used to secure the patient’s head and neck and prevent compression of the nerve by the surgeon’s elbow during surgery. Also, a special shoulder operating table is useful for improving the patient comfort and for improving body alignment. During general anesthesia, intubation should be performed atraumatically and the depth of the tube should be checked during surgery. Head misplacement can be prevented by keeping the patient conscious during surgery [
3]. For an accurate and safe nerve block, brachial plexus should be blocked by using ultrasonogram and/or nerve stimulator and minimum effective volume of local anesthetics. Also, the extent of nerve block should be checked after BPB.
In conclusion, the cause of isolated hypoglossal nerve injury in our patient was assumed to be direct compression of the hypoglossal nerve beneath the angle of the mandible by the strap used to secure the head during surgery. Also, head hyperextension to the right during shoulder surgery aggravates the nerve injury. Other risk factors for hypoglossal nerve injury may be related to airway instrument device for general anesthesia and BPB.
If cranial nerve injury is suspected, consultation with a neurologist, otolaryngologist, and psychiatrist is required. We suggest that the patient’s head should be firmly secured to the headrest, and head position should be checked frequently during shoulder surgery.

