This article has been
cited by other articles in ScienceCentral.
Abstract
Rhabdomyolysis is characterized by the breakdown of skeletal muscle and the subsequent release of intracellular contents into the circulatory system. It is potentially life-threatening because it is sometimes associated with very high creatine kinase levels, myoglobinuria, and acute renal failure. We experienced a case of postoperative rhabdomyolysis after prolonged laparoscopic radical nephrectomy in the semi-lateral decubitus position. It was associated with suspicious gluteal compartment syndrome. Fortunately, the patient’s renal function was normal through his hospital course. Rhabdomyolysis is well worth considering at the point of intraoperative positioning and postoperative care after prolonged surgery.
Go to :

Keywords: Decubitus position, Gluteal compartment syndrome, Nephrectomy, Rhabdomyolysis
INTRODUCTION
Rhabdomyolysis is defined as injury and breakdown of the skeletal muscle that results in the release of intracellular contents including creatine kinase (CK), glutamic oxalacetic transaminase (SGOT), lactate dehydrogenase (LDH), aldolase, the haeme pigment myoglobin, potassium, phosphate, and purines into the circulation [
1,
2]. Prolonged immobilization that occurs in anesthesia, coma, and drug- or alcohol-induced unconsciousness has been reported to cause rhabdomyolysis due to unrelieved pressure on gravity-dependent body parts [
3].
In this case report, we present a case of postoperative rhabdomyolysis associated with gluteal compartment syndrome after prolonged laparoscopic radical nephrectomy in the fully flexed semi-lateral decubitus position and discuss management of rhabdomyolysis and prevention of acute renal failure.
Go to :

CASE REPORT
A 54-year-old male patient 76 kg in weight and 170 cm in height (body mass index 26.3 kg/m2) was scheduled for laparoscopic radical left nephrectomy and cholecystectomy. He had received laparoscopic gastric resection twice 10 years before, a right mastoidectomy and tympanoplasty 4 years previously, and endoscopic polypectomy of the sigmoid colon 1 month earlier. All the surgeries were done under general anesthesia without any complications. He was a smoker and a diabetic for 14 years. His blood glucose values were maintained at 95–138 mg/dl after admission. Other laboratory values were normal including blood urea nitrogen 19.3 mg/dl, serum creatinine 1.02 mg/dl, SGOT 21 U/L, and glutamic pyruvate transaminase (28 U/L). His right internal jugular vein had been catheterized under ultrasonographic guidance by a radiologist the previous day.
After arriving at the operating room, initial vital signs were blood pressure 129/88 mmHg, heart rate 80 beats/min, respiratory rate 18 breaths/min, and arterial oxygen saturation 95% by pulse oximetry. We induced general anesthesia with 120 mg of propofol, supplemental remifentanil continuous infusion, and 6% desflurane, with rocuronium bromide as a muscle relaxant. After intubation of the trachea with an endotracheal tube (internal diameter 8.0 mm), we cannulated the right radial artery after performing a modified Allen test. The patient was laid in a right down, semi-lateral (30°), and fully flexed decubitus position for laparoscopic radical left nephrectomy. Adequate padding with sponge and gel pad was applied under dependent parts of the body including the axilla, hip, knee, ankle, and part of the back. Also, the kidney bridge was elevated for better kidney exposure. The pneumoperitoneum was made with the pressure limit set at 15 mmHg. The subsequent laparoscopic radical nephrectomy was not eventful, although it took 6 hours, 30 minutes. After completion of laparoscopic radical nephrectomy, the patient was placed in the supine position for laparoscopic cholecystectomy. But, the laparoscopic cholecystectomy was converted to open surgery because of serious adhesion as a result of previous distal gastric resections and anastomosis. The cholecystectomy took 2 hours. During nephrectomy and cholecystectomy, all hemodynamic parameters including invasive arterial blood pressure and central venous pressure, and respiratory parameters including arterial blood gas analysis results were maintained in the normal range. Extubation was done after complete reversal of rocuronium bromide with sugammadex 200 mg. The patient was moved to the recovery room. Recovery was uneventful. The duration of total anesthesia was 8 hours, 25 minutes. We infused 3,050 ml of crystalloid solution and 500 ml of colloid solution during operation. Estimated blood loss was 200 ml. Urine output was low (100 ml for the first 3 hours). Following administration of 20 mg of furosemide, total measured urine output was 1,000 ml by the end of the operation. Routine nursing check at the end of anesthesia revealed no abnormal finding on the body surface of the patient including the dependent region.
The next day, the patient complained of severe pain in the right buttock, which was the dependent region during laparoscopic radical nephrectomy. The pain was accompanied by swelling and hardness. Plasma CK and LDH were greatly increased to 51,050 IU/L and 2,228 IU/L, respectively (normal range 56–244 IU/L and 200–400 IU/L, respectively). Myoglobin level in spot urine testing on postoperative day 1 was 165 ng/ml and increased to 827 ng/ml by postoperative day 2. We consulted to an orthopedic physician for an evaluation of the buttock pain and swelling. The symptoms were thought to have resulted from muscle contusion of the dependent region and compression by prolonged surgery at a fixed position and resulting rhabdomyolysis. Conservative care including hydration, leg elevation and Theoesberiven-F Injection
® (Proxyphylline 240 mg with Coumarin 3 mg; Daihan Pharma Co. Ltd., Seoul, Korea) therapy to improve edema and inflammation was done, with rhabdomyolysis level checked daily (
Table 1). Urinary output was checked carefully; it was consistently 1,500–2,200 ml each day. Despite this conservative care, the swelling and bruising enlarged toward the thigh as the buttock pain decreased from postoperative day 5 onward. Magnetic resonance imaging on postoperative day 6 revealed extensive muscle edema with necrosis involving the right hip rotators and some adductors and left iliopsoas muscles combined with secondary infections (
Fig. 1). The symptoms were clinically compatible with gluteal compartment syndrome with severe rhabdomyolysis. The consulting orthopedic physician recommended use of prophylactic antibiotics and continued conservative care. The symptoms improved slowly and the patient was discharged in tolerable condition on postoperative day 9. His symptoms had completely disappeared 15 days after the operation.
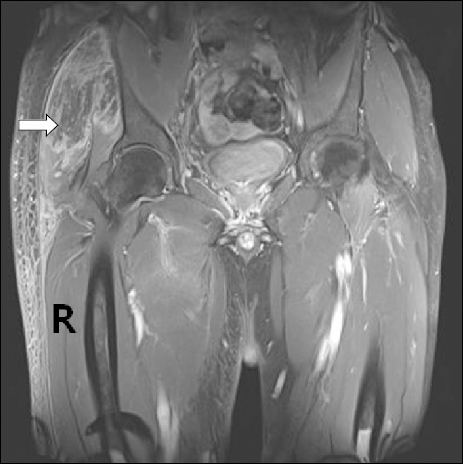 | Fig. 1MRI axial view shows extensive muscle edema with necrosis involving the right hip rotators (arrow), compared with the left side. 
|
Table 1
Sequential Laboratory Values
|
Variables (normal values) |
POD 1 |
POD 2 |
POD 3 |
POD 4 |
POD 5 |
POD 6 |
POD 7 |
|
CK (56–244 U/L) |
51050 |
38000 |
23760 |
22930 |
21800 |
16080 |
10700 |
|
LDH (200–400 IU/L) |
2228 |
2046 |
1811 |
1938 |
2171 |
2013 |
2782 |
|
SGOT (7–36 U/L) |
569 |
556 |
449 |
446 |
380 |
288 |
927 |
|
Creatinine (0.67–1.17 mg/dl) |
1.11 |
1.16 |
0.98 |
0.97 |
|
0.96 |
0.98 |
|
Cr-eGFR |
70.99 |
67.48 |
81.98 |
82.95 |
|
83.95 |
81.98 |
|
BUN (8.0–20.0 mg/dl) |
19.1 |
15.8 |
13.3 |
13.7 |
|
18 |
16 |

Go to :

DISCUSSION
Rhabdomyolysis associated with a surgical procedure involving an improper position is rare, although the most common cause was muscular trauma, and less commonly, muscle enzyme deficiencies, electrolyte abnormalities, infections, drugs, toxins, and endocrinopathies [
4,
5]. Positions that can lead to rhabdomyolysis after surgery include lateral decubitus, lithotomy, sitting, knee-to-chest and prone position [
6]. In the field of urologic surgery, rhabdomyolysis after nephrectomy in the lateral decubitus position has not been reported often [
7,
8]. For nephrectomy, the operating tables are usually flexed to orient the patient’s head and foot in the lateral decubitus position. This flank position facilitates a flank incision and exposure of kidney, but creates more pressure on the dependent region and worsens circulation in that area. Rehman et al. [
9] recommended to use little flexion of the table during nephrectomy and bring the table to a neutral position immediately after kidney retrieval. Deane et al. [
10] recommended a half-flexed flank position rather than a full-flexed flank position. It is obvious that the fully-flexed flank position we used in this case contributed to the postoperative rhabdomyolysis. If possible, it is better to use little or half-flexed flank position to avoid rhabdomyolysis.
Identification of persons at risk for rhabdomyolysis after surgery is also important. Predisposing factors of rhabdomyolysis after surgery include long operative time, increased body mass, lateral decubitus positioning, and extracellular volume depletion [
11]. Pariser et al. [
12] suggested that risk factors of rhabdomyolysis include male sex, younger age, diabetes, chronic kidney disease, and perioperative bleeding in major urologic surgery. In this case, many factors including male sex, diabetes, and increased body mass in addition to surgical factors like long operative time and fully-flexed lateral decubitus position might have contributed to the development of rhabdomyolysis.
Establishing a diagnosis of rhabdomyolysis is based primarily on the myoglobinuria or by a marked elevation in serum CK levels. Plasma myoglobin increases rapidly after muscle injury, is cleared quickly through renal excretion, and returns to normal within 24 hours. In contrast, serum CK levels rise 2–12 hours after muscle injury, peak at 3–5 days, and decline over the subsequent 6–10 days. This case showed typical changes of CK level in rhabdomyolysis. Measurement of CK levels may provide the most reliable biochemical marker of rhabdomyolysis and its severity. CK elevation 5 times the upper limit of normal is the defining biochemical abnormality for rhabdomyolysis [
13].
The principles of treatment in rhabdomyolysis include maintaining a renal protective urine output of 150–300 ml/h, consideration of urine alkalinization with sodium bicarbonate, and the use of mannitol to promote diuresis and prevent formation of casts in the kidney tubules. Also, agents that can decrease renal blood flow should be avoided [
14].
Gluteal compartment syndromes with measured compartment pressure values after surgery have been reported rarely [
15]. In our case, the dependent buttock in the semi-lateral decubitus position was more compressed and circulation was more disturbed, rather than true lateral decubitus position, resulting in gluteal compartment syndrome. Even though we did not measure compartment pressure, symptoms and clinical signs were compatible for gluteal compartment syndrome and MRI findings supported the diagnosis. Because it was thought to be a mild form, we did not perform fasciotomy and started conservative treatment. But, we thought that the gluteal compartment syndrome was associated with position and was preventable by adequate padding and positioning.
In conclusion, clinicians have to pay particular attention to adequate positioning to avoid rhabdomyolysis. In the lateral decubitus position, little or half-flexion of the table, avoiding use of kidney bridge, and neutral positioning after the main procedure are recommended. Also, the risk factors of rhabdomyolysis, which include prolonged surgery, increased body mass, male sex, younger age, diabetes, chronic kidney disease, and perioperative bleeding, should be kept in mind.
Go to :


