Abstract
Background:
Long-chain triglyceride/medium-chain triglyceride (LCT/MCT) propofol is less painful than standard long chain triglyceride (LCT) propofol; however, there is still a need to reduce severe pain. 5-Hydroxytryptamine type 3 (5-HT3) receptor antagonists have an analgesic effect during the injection of standard LCT propofol. This study compared the incidence of moderate-to-severe injection pain with LCT/MCT propofol using pretreatment with palonosetron versus pretreatment with saline or lidocaine.
Methods:
This prospective, randomized double-blind study divided 98 patients scheduled for general anesthesia into three groups: control (n = 33), palonosetron (n = 32), and lidocaine (n = 33) groups. An 18-gauge intravenous catheter was inserted into the peripheral vein at the dorsum of the hand. The patient’s vein was occluded by a rubber tourniquet at mid-forearm, and we then administered 2 ml of the pretreatment drug. One minute after venous occlusion, we released the tourniquet and administered LCT/MCT propofol 2 mg/kg for 10–15 seconds. The degree of pain on propofol injection was evaluated using a 4-point scale.
Go to : 
Recently, there has been gradually increasing interest in the comfort and satisfaction of perioperative patients. Propofol is among the most popular intravenous anesthetic agents because it provides rapid induction and recovery, good quality anesthesia without any “hangover,” and an antiemetic effect [1]. Despite numerous advantages, propofol has the disadvantage of inducing pain during injection, in up to 70% of cases without pretreatment [2,3]. Pain during injection is the seventh-most important and frequent problem in American clinical anesthesia and may be a cause of patient dissatisfaction with anesthesia [4].
Long-chain triglyceride/medium-chain triglyceride (LCT/MCT) propofol causes less pain than standard long-chain triglyceride (LCT) propofol due to the decreased free propofol concentration in the emulsion in the aqueous phase [5]. Many researchers have reported that LCT/MCT propofol significantly reduced propofol injection pain [6-10]. Nevertheless, 24–63% of patients still suffer from injection pain with LCT/MCT propofol [10,11]. Moreover, Sundarathiti et al. [12] reported that 36.4% of patients have moderate to severe pain on LCT/MCT propofol injection. Therefore, there is still need to study the prevention of LCT/MCT propofol injection pain.
Various methods for reducing propofol injection pain have been reported, of which pretreatment with lidocaine with venous occlusion is the most effective [2,3]. A recent study reported that lidocaine pretreatment and lidocaine admixture for propofol injection pain have similar effects [13].
5-Hydroxytryptamine type 3 (5-HT3) receptor antagonists such as ondansetron exert strong local anesthetic effects and actions on the μ receptor, and have been reported to lessen propofol injection pain [14-16]. In addition, as strong antiemetics, they have the advantage of reducing postoperative nausea and vomiting.
Palonosetron, the newest 5-HT3 antagonist, has a strong affinity for 5-HT3 receptors and a long-duration antiemetic effect. Two studies have examined the use of palonosetron to reduce propofol injection pain. Ryu and Kim [17] reported that palonosetron reduced the incidence of propofol injection pain, while Lee et al. [18] reported that palonosetron did not reduce the incidence of propofol injection pain, but it did reduce severe pain. These differing results have been attributed to the use of different methods or propofol formulations. Therefore, this study evaluated the ability of palonosetron to prevent pain on injection of LCT/MCT propofol.
Go to : 
This study used a planned, prospective, randomized double-blind design that was approved by our hospital’s Institutional Review Board. Ninety-nine patients scheduled for elective surgery under general anesthesia were included. They were between 19 and 70 years of age and American Society of Anesthesiologists (ASA) classification I or II. The exclusion criteria were allergy to propofol, palonosetron, or lidocaine; peripheral vascular or neurologic disease; use of analgesics or sedatives within the 24 h before surgery; dementia or communication difficulties; and pregnancy. All patients provided written informed consent, and they were divided randomly by a computer using block randomization into control (0.9% normal saline 2 ml, n = 33), palonosetron (palonosetron 0.075 mg/2 ml, n = 33), and lidocaine (2% lidocaine 40 mg/2 ml, n = 33) groups.
Pretreatment drugs (2 ml solution) were prepared in identical syringes by an independent anesthesiologist not involved in the study. The treating anesthesiologist was blinded to the pretreatment drug administered to each subject.
All patients were administered glycopyrrolate 0.2 mg intramuscularly 30 minutes before surgery. On arrival in the operating room, an 18-gauge cannula was inserted into the dorsal vein of the hand, and peripheral oxygen saturation was monitored by pulse oximetry; electrocardiography and non-invasive blood pressure measurement were also initiated. The dorsal vein was occluded by a rubber tourniquet at mid-forearm; we then administered 2 ml of the pretreatment drug. After venous occlusion by the rubber tourniquet for 1 minute, we released the tourniquet and administered LCT/MCT propofol (Fresofol 1%, Fresenius Kabi, Graz, Austria) 2 mg/kg for 10–15 seconds.
Pain assessment was performed from the time of injection of propofol until loss of consciousness by an independent anesthesiologist who was blinded to the patients’ group assignment. The highest pain scores during this period were recorded. All patients were asked the following question: “Do you have any pain or discomfort in your arm during injection of hypnotics?” and the degree of pain from the propofol injection was measured using the 4-point scale proposed by McCririck and Hunter (0 = none, 1 = mild pain, 2 = moderate pain, 3 = severe pain [Table 1]) [19]. Pain or discomfort on injection of the pretreatment drug, and any adverse effects of palonosetron, were recorded.
Assessment of Pain
Thereafter, patients were administered rocuronium for muscle relaxation, and an endotracheal tube was inserted. Then, the operation began.
The primary endpoint was the incidence of moderate-to-severe pain on injection of propofol due to its clinical importance; secondary endpoints were overall pain incidence and other complications.
The estimated sample size was calculated based on the difference between moderate-to-severe pain incidence (35 vs. 2.5%) reported by Ryu and Kim [17] (α = 0.05/3, β = 0.2, dropout rate 20%); it was calculated that 33 patients were required per group. Statistical analyses were performed using SPSS for Windows (ver. 14.0, SPSS, Chicago, IL, USA). The groups were compared using one-way ANOVA for continuous data, with the chi-square test, Fisher’s exact test, or a generalized linear mixed model applied for categorical data. All data are presented as the means ± SD for continuous data, and as frequencies with percent for categorical data. A value of P < 0.05 was taken to indicate statistical significance.
Go to : 
Of the 99 patients enrolled, one patient in the palonosetron group was excluded due to preoperative use of an uninformed analgesic drug. A total of 98 patients were included in this study: control group, n = 33; palonosetron group, n = 32; and lidocaine group, n = 33. Baseline characteristics including age, sex ratio, weight, and ASA classification were similar among groups (Table 2).
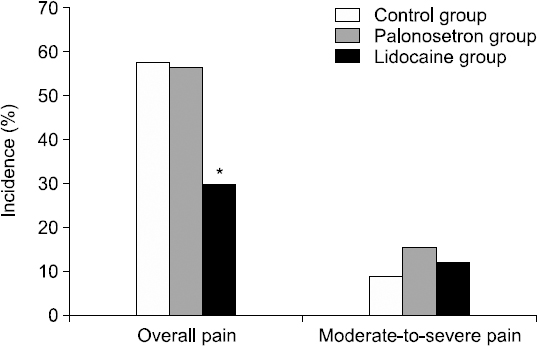
The number of patients who exhibited moderate-to-severe pain was similar between the three groups: three patients (9.1%) in the control group, five patients (15.6%) in the palonosetron group, and four patients (12.1%) in the lidocaine group (P = 0.693; Fig. 1).
The overall incidence of pain differed among the three groups, i.e., 19 patients (57%) in the control group, 18 patients (56.3%) in the palonosetron group, and 10 patients (30.3%) in the lidocaine group (P = 0.044, chi-square test; Fig. 1). The overall incidence of pain was lower in the lidocaine group compared with the control and palonosetron groups (P = 0.028, generalized linear mixed model).
Table 3 presents the distribution of propofol injection pain; there were no significant group differences (P = 0.076).
Distribution of Propofol Injection Pain
No patients complained of pain during injection of the pretreatment drug and none had any adverse effects after injection of palonosetron and propofol.
Go to : 
This study demonstrated that palonosetron pretreatment with venous occlusion does not reduce the incidence of moderate-to-severe or overall pain on injection of 1% LCT/MCT propofol compared with control pretreatment. Lidocaine pretreatment with venous occlusion does not reduce moderate-to-severe pain, although it reduces the overall incidence of propofol injection pain.
The reported incidence of propofol injection pain on the dorsum of the hand without pretreatment is 28–90% [2,3,20]. The mechanism of propofol injection pain remains unclear, but it has been suggested that the initial pain is due to the direct stimulation of free nerve endings and nociceptors in the blood vessel [20], and is related to the free drug concentration in the aqueous phase of the emulsion and lipid carrier [5,21]. Delayed pain occurs within 30 seconds of propofol injection, and results from indirect effects via the kinin cascade [20]. It has been suggested that it may be associated with activation of the kallikrein-kinin pathway, which is related to hyper-permeability and vasodilatation by caused bradykinin and prostaglandin E2 [22,23].
Several studies have reported pharmacologic and non-pharmacologic methods for reducing propofol injection pain [20,24], including the use of a larger vein, changes in the infusion rate, dilution or cooling of the drug, and the use of pretreatment drugs (opioids, ketamine, non-steroidal anti-inflammatory drugs, 5-HT3 antagonists, lidocaine), a lidocaine-propofol admixture, or LCT/MCT propofol [2,3,24].
Lidocaine pretreatment with venous occlusion and the use of a larger vessel, are the most effective methods of reducing pain, while lidocaine pretreatment is the most popular method [2,3]. The mechanism of action of lidocaine pretreatment with venous occlusion may be associated with its local anesthetic and stabilizing effects on the kinin cascade [25]. Our results also showed that lidocaine pretreatment reduced the incidence of propofol injection pain.
In an animal study, Ye et al. [16] found that ondansetron had 15-times the local anesthetic potential of lidocaine. Agonist activity at the μ-opioid receptor was also demonstrated [15]. In addition, the 5-HT3 receptor is associated with the release of pain mediators, such as substance P, in nerve terminals, and 5-HT3 antagonists have been reported to affect substance P-mediated inflammation and hyperalgesia [26]. Through these multifactorial actions, several 5-HT3 antagonists have been shown to reduce propofol injection pain compared with placebo. Ambesh et al. [14] reported that ondansetron reduced the incidence of pain and severe pain associated with propofol injection relative to placebo (incidence: 25 vs. 55%; severe pain: 7.5 vs. 32.5%). Ahmed et al. [27] reported that granisetron decreased the overall incidence of pain and severe pain on injection of propofol compared with placebo (incidence: 15 vs. 60%, severe pain: 2.5 vs. 37.5%). Lee et al. [28] reported that ramosetron decreased the incidence of injection pain caused by microemulsion propofol, from 96 to 60%.
Palonosetron is a novel 5-HT3 receptor antagonist that has a unique molecular structure and pharmacokinetic profile compared with other 5-HT3 receptor antagonists, including a longer half-life and greater binding affinity [29]. As a result, palonosetron is more effective than ondansetron for preventing delayed postoperative nausea and vomiting [30]. In our hospital, palonosetron is commonly used as an antiemetic drug for the prevention of postoperative nausea and vomiting.
Recently, Ryu and Kim [17] reported that palonosetron effectively reduced the propofol injection pain from 60 to 27.5% compared with a control group; furthermore, no patient in the palonosetron group complained of severe pain. Lee et al. [18] reported that palonosetron did not reduce the overall incidence of propofol injection pain, although it reduced the incidence of severe pain from 33% to 3% compared with a control group.
Our results showed that palonosetron did not reduce the incidence of overall pain or moderate-to-severe pain on propofol injection. We suggest the following three reasons for the differences in the results of these studies.
First, the different studies used different formulations of propofol. Our study used LCT/MCT propofol, which is reported to cause less pain than standard LCT propofol due to a decreased free propofol concentration in the emulsion during the aqueous phase [5]. LCT/MCT propofol, which is made by mixing medium chain triglyceride in standard LCT propofol, has a 24.5% decreased free propofol concentration in the emulsion in the aqueous phase compared with standard LCT propofol [5]. As a result, decreased incidence and severity of propofol injection pain, while maintaining the pharmacological properties of standard LCT propofol, has been reported for LCT/MCT propofol [7]. Larsen et al. [9] reported that LCT/MCT propofol decreased the incidence of injection pain from 64 to 37% relative to LCT propofol. Yew et al. [10] reported that the injection pain associated with LCT/MCT propofol is similar to the pain associated with a lidocaine-LCT propofol admixture (24%). Furthermore, the lidocaine-LCT/MCT propofol admixture confers an additional reduction effect on injection pain, from 24 to 4%. Kam et al. [8] reported that the pain-free ratio of LCT/MCT propofol was similar to that of a lidocaine-LCT propofol admixture (38 vs. 36% respectively), and no patient complained of severe pain.
Ryu and Kim [17] used LCT propofol (Provive™, Myungmoon Pharm., Seoul, Korea). Lee et al. [18] used total intravenous anesthesia with effect-site target-controlled infusion (TCI) using a TCI pump, although they did not state the formulation used. In our study, we suggest that the use of LCT/MCT propofol was responsible for the lack of group differences in moderate-to-severe pain; it may have reduced pain severity in all groups.
Second, the doses used were different. We used a full dose of propofol, unlike a study that used a 25% dose, for the evaluation of pain scores [17]. The use of a reduced dose of propofol confers a benefit in that it is possible to measure pain scores when patients are awake, but scores may be reduced because the concentration to which the vessel wall is exposed is reduced. We used a full dose of propofol for induction because evaluating the pain encountered in a real clinical situation is clinically important. However, our method of using a full dose has the disadvantage of rendering the evaluation of pain scores difficult because patients fall asleep within 1 minute. In our study, when propofol was administered, patients initially felt a cool sensation along the vein, and then complained of pain about 20 seconds after injection. Therefore, pain scores for delayed pain can be underestimated because patients fall asleep rapidly. However, our pain scores may have been relatively accurate because we evaluated pain based not only on patients’ answers but also with respect to pain behaviors such as arm movements; furthermore, our test conditions were close to the real clinical situation of propofol injection pain. We also suggest that the use of the total dose explains why there were no group differences in the incidence of moderate-to-severe pain in our study.
Third, the methods used were different. Lee et al. [18] used total intravenous anesthesia with effect-site TCI, via a TCI pump; their dose and speed of injection may have been different from ours. This may be another reason for the different result.
Using LCT/MCT propofol, the clinical impact of propofol injection pain has been markedly reduced. Allford and Mensah [6] reported that moderate-to-severe pain on LCT/MCT propofol injection was reduced by 54% compared with conventional LCT propofol. However, 24–63% of patients still suffer from injection pain with LCT/MCT propofol [10,11]. Moreover, Sundarathiti et al. [12] reported that 36.4% of patients have moderate-to-severe pain (VAS > 4) on LCT/MCT propofol injection. Therefore, we believe that further research on LCT/MCT propofol injection pain is needed.
Our study has a critical limitation in terms of sample size because the propofol formulation used in this study was assumed to be equal to that of the previous study that we used for calculating the sample size. It is possible that the result of the current study has low power and type II error due to the relatively small sample size. Further study is needed to prove the effect of these drugs on pain on injection of LCT/MCT propofol with a larger sample size.
In conclusion, palonosetron pretreatment does not reduce the incidence and severity of pain on injection of 1% LCT/MCT propofol. Lidocaine pretreatment does not reduce propofol injection pain severity, although it reduces the overall incidence of propofol injection pain.
Go to : 
REFERENCES
1. Bryson HM, Fulton BR, Faulds D. Propofol. An update of its use in anaesthesia and conscious sedation. Drugs. 1995; 50:513–59. DOI: 10.2165/00003495-199550030-00008. PMID: 8521772.
2. Jalota L, Kalira V, George E, Shi YY, Hornuss C, Radke O, et al. Prevention of pain on injection of propofol: systematic review and meta-analysis. BMJ. 2011; 342:d1110. DOI: 10.1136/bmj.d1110. PMID: 21406529.

3. Picard P, Tramèr MR. Prevention of pain on injection with propofol: a quantitative systematic review. Anesth Analg. 2000; 90:963–9. DOI: 10.1097/00000539-200004000-00035. PMID: 10735808.

4. Macario A, Weinger M, Truong P, Lee M. Which clinical anesthesia outcomes are both common and important to avoid? The perspective of a panel of expert anesthesiologists. Anesth Analg. 1999; 88:1085–91. DOI: 10.1213/00000539-199905000-00023. PMID: 10320175.

5. Doenicke AW, Roizen MF, Rau J, Kellermann W, Babl J. Reducing pain during propofol injection: the role of the solvent. Anesth Analg. 1996; 82:472–4. DOI: 10.1213/00000539-199603000-00007. PMID: 8623945.
6. Allford MA, Mensah JA. Discomfort on injection: a comparison between two formulations of propofol. Eur J Anaesthesiol. 2006; 23:971–4. DOI: 10.1017/S0265021506001049. PMID: 16824242.
7. Doenicke AW, Roizen MF, Rau J, O’Connor M, Kugler J, Klotz U, et al. Pharmacokinetics and pharmacodynamics of propofol in a new solvent. Anesth Analg. 1997; 85:1399–403. DOI: 10.1097/00000539-199712000-00040. PMID: 9390616.

8. Kam E, Abdul-Latif MS, McCluskey A. Comparison of Propofol-Lipuro with propofol mixed with lidocaine 10 mg on propofol injection pain. Anaesthesia. 2004; 59:1167–9. DOI: 10.1111/j.1365-2044.2004.03964.x. PMID: 15549974.

9. Larsen B, Beerhalter U, Biedler A, Brandt A, Doege F, Brün K, et al. Less pain on injection by a new formulation of propofol? A comparison with propofol LCT. Anaesthesist. 2001; 50:842–5. DOI: 10.1007/s00101-001-0234-0. PMID: 11760478.
10. Yew WS, Chong SY, Tan KH, Goh MH. The effects of intravenous lidocaine on pain during injection of medium- and long-chain triglyceride propofol emulsions. Anesth Analg. 2005; 100:1693–5. DOI: 10.1213/01.ANE.0000151718.58709.0B. PMID: 15920197.

11. Sethi N, Jayaraman L, Sethi M, Sharma S, Sood J. Prevention of propofol pain: a comparative study. Middle East J Anaesthesiol. 2009; 20:71–4. PMID: 19266829.
12. Sundarathiti P, Boonthom N, Chalacheewa T, Jommaroeng P, Rungsithiwan W. A comparison of propofol-LCT with propofol-LCT/MCT on pain of injection. J Med Assoc Thai. 2007; 90:2683–8. PMID: 18386721.
13. Euasobhon P, Dej-Arkom S, Siriussawakul A, Muangman S, Sriraj W, Pattanittum P, et al. Lidocaine for reducing propofol-induced pain on induction of anaesthesia in adults. Cochrane Database Syst Rev. 2016; 2:CD007874. DOI: 10.1002/14651858.cd007874.pub2.

14. Ambesh SP, Dubey PK, Sinha PK. Ondansetron pretreatment to alleviate pain on propofol injection: a randomized, controlled, double-blinded study. Anesth Analg. 1999; 89:197–9. DOI: 10.1097/00000539-199907000-00035. PMID: 10389803.
15. Gregory RE, Ettinger DS. 5-HT3 receptor antagonists for the prevention of chemotherapy-induced nausea and vomiting. A comparison of their pharmacology and clinical efficacy. Drugs. 1998; 55:173–89. DOI: 10.2165/00003495-199855020-00002. PMID: 9506240.
16. Ye JH, Mui WC, Ren J, Hunt TE, Wu WH, Zbuzek VK. Ondansetron exhibits the properties of a local anesthetic. Anesth Analg. 1997; 85:1116–21. DOI: 10.1213/00000539-199711000-00029.

17. Ryu HB, Kim SJ. Analgesic effects of palonosetron in the intravenous propofol injection. Korean J Anesthesiol. 2014; 66:99–104. DOI: 10.4097/kjae.2014.66.2.99. PMID: 24624266. PMCID: PMC3948450.

18. Lee KH, Rim SK, Lee JY, Lee SY, Lee SN, Lee EJ, et al. Effects of pretreatment with intravenous palonosetron for propofol-remifentanil-based anesthesia in breast and thyroid cancer surgery: a double-blind, randomized, controlled study. Korean J Anesthesiol. 2014; 67:13–9. DOI: 10.4097/kjae.2014.67.1.13. PMID: 25097733. PMCID: PMC4121488.

19. McCrirrick A, Hunter S. Pain on injection of propofol: the effect of injectate temperature. Anaesthesia. 1990; 45:443–4. DOI: 10.1111/j.1365-2044.1990.tb14329.x. PMID: 2200300.

20. Tan CH, Onsiong MK. Pain on injection of propofol. Anaesthesia. 1998; 53:468–76. DOI: 10.1046/j.1365-2044.1998.00405.x.

21. Klement W, Arndt JO. Pain on injection of propofol: effects of concentration and diluent. Br J Anaesth. 1991; 67:281–4. DOI: 10.1093/bja/67.3.281. PMID: 1911014.

22. Ando R, Watanabe C. Characteristics of propofol-evoked vascular pain in anaesthetized rats. Br J Anaesth. 2005; 95:384–92. DOI: 10.1093/bja/aei184. PMID: 15994849.

23. Nakane M, Iwama H. A potential mechanism of propofol-induced pain on injection based on studies using nafamostat mesilate. Br J Anaesth. 1999; 83:397–404. DOI: 10.1093/bja/83.3.397. PMID: 10655909.

24. Nam YM, Byun JW, Kwon GM, Kim YO, Lee JM, Jeon WJ. Reduction of propofol infusion pain using target controlled infusion of remifentanil. Anesth Pain Med. 2014; 9:36–40.
25. Scott RP, Saunders DA, Norman J. Propofol: clinical strategies for preventing the pain of injection. Anaesthesia. 1988; 43:492–4. DOI: 10.1111/j.1365-2044.1988.tb06641.x. PMID: 3261547.

26. Faerber L, Drechsler S, Ladenburger S, Gschaidmeier H, Fischer W. The neuronal 5-HT3 receptor network after 20 years of research--evolving concepts in management of pain and inflammation. Eur J Pharmacol. 2007; 560:1–8. DOI: 10.1016/j.ejphar.2007.01.028. PMID: 17316606.
27. Ahmed A, Sengupta S, Das T, Rudra A, Iqbal A. Pre-treatment with intravenous granisetron to alleviate pain on propofol injection: A double-blind, randomized, controlled trial. Indian J Anaesth. 2012; 56:135–8. DOI: 10.4103/0019-5049.96308. PMID: 22701203. PMCID: PMC3371487.

28. Lee HY, Kim SH, So KY. Prevention of microemulsion propofol injection pain: a comparison of a combination of lidocaine and ramosetron with lidocaine or ramosetron alone. Korean J Anesthesiol. 2011; 61:30–4. DOI: 10.4097/kjae.2011.61.1.30. PMID: 21860748. PMCID: PMC3155134.

29. Stoltz R, Cyong JC, Shah A, Parisi S. Pharmacokinetic and safety evaluation of palonosetron, a 5-hydroxytryptamine-3 receptor antagonist, in U.S. and Japanese healthy subjects. J Clin Pharmacol. 2004; 44:520–31. DOI: 10.1177/0091270004264641. PMID: 15102873.

30. Moon YE, Joo J, Kim JE, Lee Y. Anti-emetic effect of ondansetron and palonosetron in thyroidectomy: a prospective, randomized, double-blind study. Br J Anaesth. 2012; 108:417–22. DOI: 10.1093/bja/aer423. PMID: 22277663.

Go to : 




 PDF
PDF Citation
Citation Print
Print


 XML Download
XML Download