INTRODUCTION
Core body temperature (T
C) can decrease during general anesthesia and surgery [
1]. Various factors including low ambient temperature [
2], cold intravenous fluids, open abdominal surgery [
3], drug induced vasodilation [
2], and the inspiration of cold fresh gas can decrease T
C [
4], which can contribute to perioperative complications such as delayed awakening and coagulopathy [
5,
6]. Particularly in elderly patients, heat production can decrease and vasoconstrictor response to a cold stress can be attenuated during surgery [
7,
8]. This can lead to decreased T
C; therefore, careful consideration is required to maintain temperature homeostasis during general anesthesia in elderly patients [
9].
Many active warming devices, such as a forced-air warming system and water-circulating mattress, are used to prevent intraoperative T
C decrease [
10,
11]. Electrically heated humidifiers (HH) can deliver warmed gas directly to the lower respiratory tract. Compared to a heat and moisture exchanger (HME), which is widely used during mechanical ventilation, HH may provide additional heat energy, thereby being more effective for maintaining T
C [
12-
15]. However, previous studies comparing HH to a conventional circuit without HME [
12] were limited by use of aural canal temperature, which is not representative of T
C, [
13] or by being performed in specialized surgical conditions such as liver transplantation [
15]. Therefore, the effect of HH, compared to HME, on intraoperative T
C decrease has not been well defined, particularly in elderly patients under general anesthesia.
Because HH can provide direct heat energy, we thought that using HH would be more effective in preventing TC decrease than using HME. To evaluate the effect of both devices on intraoperative TC change, we serially measured TC at specific time-points and investigated the change in TC from baseline.
Go to :

MATERIALS AND METHODS
After obtaining approval from our Institutional Review Board and registering with Clinical Research Information Service (registration number: KCT0000910), this study was conducted between March 2013 and December 2013. A total of 24 elderly patients undergoing open urologic surgery were enrolled after obtaining written informed consent. All patients were ASA physical status 1 or 2, and patients with any history of severe cardiopulmonary disease, abnormal pulmonary function on preoperative testing, obese patients (body mass index, BMI > 30 kg/m2), thin patients (BMI < 18.5 kg/m2), and those with abnormal preoperative temperature (> 38°C or < 36°C) were excluded. All patients were evaluated with standard preoperative work-up examinations at our institution. None of the patients were premedicated.
After admission for surgery, all patients were randomly assigned into one of two groups by using computer-generated random numbers. Mechanical ventilation was achieved using a conventional ventilator circuit with HME in group C (n = 12) or using a HH in group H (n = 12). In group C, HME (Humid Vent, Gibeck, Sweden), was placed between the tracheal tube and a Y-piece of the ventilatory circuit. In group H, HH (ANAPOD™, Westmed Inc. Tucson, AZ, USA) was used to deliver warmed and humidified inspiratory gas. HH consisted of an electrically heated wire that was set to 38°C by a servo-control system and water-absorbing sponge in the inspiratory limb.
In accordance with our institutional standards, the same intraoperative patient monitoring protocol was applied in both groups. General anesthesia was induced with thiopental sodium 5 mg/kg, and vecuronium 0.15 mg/kg was used to facilitate tracheal intubation. Anesthesia was maintained by administering 1–3 vol% sevoflurane and 2 L/min of 50% oxygen and medical air mixture. The depth of anesthesia was monitored using the bispectral index (BIS A-1050 Monitor, Aspect Medical Systems, Newton, MA) and was maintained between 40–60 during surgery. Patients were connected to the anesthesia machine (Primus®; Drägerwerk AG & Co., Lübeck, Germany) and ventilated using a tidal volume of 6–8 ml/kg of the patient’s ideal body weight and 8 cmH2O of positive end-expiratory pressure. The respiratory rate was adjusted to maintain normocapnia. Administered intravenous fluid was warmed to 37°C by a fluid warming device, and packed red blood cells were administered at ambient temperature. During surgery, mean blood pressure was maintained > 65 mmHg and heart rate at < 110 beats/min. Transfusion was initiated when the intraoperative hemoglobin level was < 8 g/dl. Patients were aggressively warmed by forced-air warming system (Bair Hugger™, 3M, St. Paul, MN, USA) when TC decreased < 35.5°C or after 150 minutes of skin incision.
Outcome measurement
Temperatures were measured at three different sites (esophagus, aural canal, and right forearm) during surgery. Esophagus temperature, considered as the TC, was continuously measured using an esophageal stethoscope with temperature sensor (Esophageal Stethoscope; DeRoyal Inc., Powell, TN, USA). An esophageal stethoscope was placed at the site where the heartbeat was best heard. Skin temperature (TS) was measured using an attachable probe (Skin temperature probe; Datex-Ohmeda, Helsinki, Finland) that was placed at the center of the anterior aspect of the right forearm. The right forearm of each patient was exposed to ambient environment during the intraoperative period.
To measure the primary outcomes, an esophageal probe was inserted immediately after tracheal intubation to the depth of the maximal cardiac sounds on auscultation. TC was recorded immediately after intubation as the baseline reading. After baseline values were measured, TC was repeatedly recorded at 15, 30, 45, 60, 90, 120, and 150 minutes after skin incision, and at the end of surgery. At each time-point, TS was monitored to assess the peripheral vasodilation occurring at the initial phase of TC decrease. Each TS measurement was performed simultaneously with the corresponding TC measurement.
For other outcomes, we assessed the amount of administered fluid and transfusion incidence. Additionally, we evaluated temperature, the amount of administered opioid, and the incidence of shivering at the postanesthesia care unit.
Statistical analysis
In a previous pilot study, the mean TC decrease from baseline 150 minutes after skin incision was 1.3°C ± 0.5°C in patients using HME and 0.7°C ± 0.5°C in patients using HH. Assuming a type I error of 0.05 and a desired power of 0.80 to test the alternative hypothesis that TC decrease was different between group H and group C, 12 patients were required for the present analysis. All data were expressed as numbers (%), the median [interquartile range], or the mean ± standard deviation (SD). Statistical analyses were performed using IBM SPSS 21.0 (IBM Corp., Armonk, NY, USA). The Shapiro-Wilk test was used to test the normality of the data. Repeated measure analysis of variance was performed to evaluate TC changes in each group. The Mann-Whitney rank-sum test was used to compare temperature between the two groups at each time-point. The student’s t-test was used to compare linear data, and Fisher’s exact test or the chi-square test was used to compare categorical data between groups. A P < 0.05 is considered to be statistically significant.
Go to :

RESULTS
A total of 24 patients were enrolled, and none dropped out during the course of the study. Demographic data of patients are presented in
Table 1. No significant differences were seen in demographics between group C and group H. Intraoperative and postoperative data are listed in
Table 2. There were no significant differences in intraoperative data and postoperative outcomes between the two groups.
Table 1
Patient Demographics and Type of Surgery
|
Variables |
Group C (n = 12) |
Group H (n = 12) |
P value |
|
Age (yr) |
70.3 ± 3.8 |
68.3 ± 2.6 |
0.15 |
|
Body mass index (kg/m2) |
23.6 ± 2.7 |
24.3 ± 3.4 |
0.56 |
|
Duration of anesthesia (min) |
376.9 ± 134.6 |
351.6 ± 109.5 |
0.62 |
|
Ambient room temperature (°C) |
23.9 ± 0.8 |
23.9 ± 0.7 |
0.98 |
|
Type of surgery |
|
|
|
|
Radical cystectomy |
9 (75%) |
7 (58%) |
0.65 |
|
Radical retropubic prostatectomy |
3 (25%) |
3 (25%) |
0.33 |
|
Radical Nephrectomy (supine position) |
0 (0%) |
2 (17%) |
0.45 |

Table 2
Intraoperative Data and Postoperative Outcomes
|
Variables |
Group C (n = 12) |
Group H (n = 12) |
P value |
|
Intraoperative data |
|
MAC-hr |
5.9 ± 2.5 |
5.2 ± 1.3 |
0.58 |
|
Awake time (seconds) |
568.7 ± 258.5 |
440.8 ± 204.3 |
0.19 |
|
Crystalloid administered (ml) |
4,200 ± 2,106 |
2,975 ± 1,268 |
0.09 |
|
Transfusion incidence |
7 (58.3%) |
3 (25.0%) |
0.21 |
|
Packed RBCs administered (unit) |
1.5 ± 1.4 |
0.4 ± 0.8 |
0.03 |
|
Postoperative outcomes |
|
Temperature at PACU (°C) |
36.3 ± 0.4 |
36.3 ± 0.3 |
0.65 |
|
Opioid administered (μg) |
65.4 ± 28.7 |
75.0 ± 39.9 |
0.51 |
|
Shivering at PACU |
1 (8.3%) |
1 (8.3%) |
|

Changes in T
C during the entire measurement period are presented in
Fig. 1. T
S was similarly changed in both groups, with no significant difference in any time-point between group C and group H (
Fig. 2, P = 0.919). In group C, T
C was significantly lower at 90, 120, and 150 minutes after skin incision compared to baseline (P < 0.001), while in group H, there was no significant T
C decrease until the end of surgery (P = 0.46). Between group C and group H, T
C differed at 60, 90, 120, and 150 minutes after skin incision (−0.6 [−0.7–−0.4]°C vs. −0.3 [−0.5–−0.1]°C, P = 0.022; −0.7 [−0.9–−0.5]°C vs. −0.4 [−0.7–−0.1]°C, P = 0.015; −0.9 [−1.1–−0.6]°C vs. −0.4 [−0.5–−0.2]°C, P = 0.006; and −1.0 [−1.3–−0.7]°C vs. −0.5 [−0.5–−0.1]°C, P = 0.013, respectively).
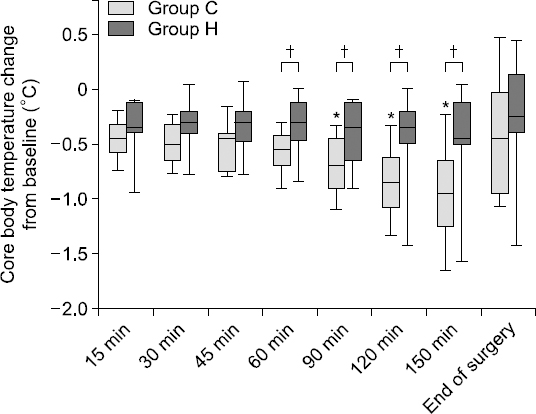 | Fig. 1Core body temperature change in group C and group H. In group C (using heat moisture exchanger, light-gray box), the core body temperature significantly decreased compared to the baseline values at 90, 120, and 150 minutes after skin incision, but in group H (using heated humidifier, dark gray box), the core body temperature did not significantly change at these time-points compared to baseline. In the vertical box-plot, the median value is indicated by a horizontal line in the box, and the upper/lower ends of the box indicate the interquartile range. *P < 0.05, compared to the baseline value, †P < 0.05, compared between group C and group H. 
|
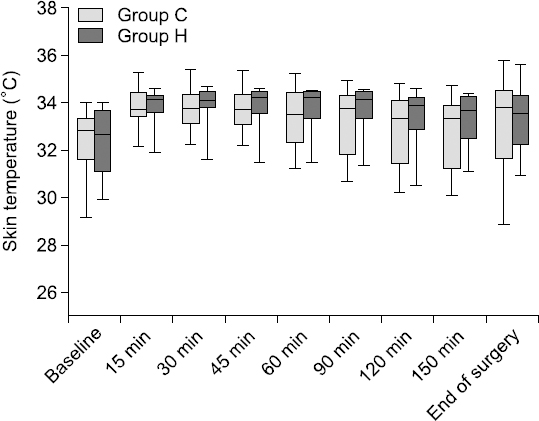 | Fig. 2Intraoperative skin temperature in group C and group H. Between group C (using heat moisture exchanger, light-gray box) and group H (using heated humidifier, dark gray box), there were no differences in skin temperature at any time-point. In the vertical box-plot, the median value is indicated by a horizontal line in the box, and the upper/lower ends of the box indicate the interquartile range. 
|
Go to :

DISCUSSION
In our present study, the TC decreased significantly in patients using HME, but did not change in patients using HH. Between the two groups, the difference in TC change became significant 90 minutes after skin incision, whilst the TS showed similar values across time points. These findings suggested a role for HH in intraoperative temperature management.
HME was effective in optimizing inspiratory gas, but had no beneficial effect on T
C change during general anesthesia [
16]. However, HH can directly supply convective heat energy and humidified gas to the tracheobronchial tree, which consequently reduces evaporation from mucosal surfaces. The proportion of heat loss from the respiratory tract is known to be < 10% of the total intraoperative heat loss [
17], but the large surface area of tracheobronchial tree may allow for effective delivery of heat energy from warmed inspiratory gas. Moreover, heat loss from mucosal vaporization can decrease > 50% in fully humidified gas [
17], which may also contribute to decreased heat loss. Therefore, despite the relatively small proportion of total intraoperative heat loss, directly providing heat energy to the lower respiratory tract via HH may effectively limit T
C decrease to < 0.5°C.
Intraoperative T
C is known to decrease in two different phases. After anesthesia is induced, T
C decreases quickly via core-to-peripheral heat redistribution by anesthetic-induced peripheral vasodilation (phase 1) [
1,
18-
20]. Then, T
C decreases slowly by heat loss via convection and radiation (phase 2) [
14,
20]. In phase 1, anesthetic-induced peripheral vasodilation can make prompt heat redistribution while preserving body heat content constantly and result in T
S increase [
21]. Our data showed that the T
S change during surgery was similar between group H and group C, suggesting that initial peripheral vasodilation did not differ between these groups. In phase 2, the negative balance between heat production and loss was a major cause of T
C decrease [
1], and peripheral vasodilation in phase 1 subsequently can also contribute to T
C decrease [
22]. In elderly patients, decreases in both heat production and thermoregulated vasoconstriction may be responsible for the increased risk of intraoperative hypothermia [
9]. Thus, we hypothesized that the role of HH on T
C decrease is more important in phase 2 than in phase 1 when the ability of heat production is compromised. HH can provide heated inspiratory gas, thereby reducing the temperature gradient between inspiratory and expiratory airflow during mechanical ventilation. Moreover, by maintaining optimal relative humidity, HH may contribute to preventing heat loss via mucosal evaporation. In this regard, by reducing heat loss in the lower respiratory tract, HH may have a superior effect on maintaining T
C, particularly in phase 2. In our current study, T
C was maintained in group H even at 90 minutes after skin incision, but was significantly decreased in group C.
Intraoperative hypothermia has been associated with increased perioperative morbidity including delayed emergence and increased blood loss [
5]. Particularly in elderly patients with decreased thermoregulatory response and drug metabolism, inadvertent hypothermia can cause serious postoperative complications [
9,
23]. However, our present data showed that emergence time, defined as the time from discontinuation of volatile anesthetics to the first response to a verbal command, was not statistically different between the two groups. Mild intraoperative hypothermia can inhibit platelet aggregation, thereby increasing risk of transfusion [
13]. Although the fact that HH is associated with decreased transfusion [
12,
24,
25], and that our data showed a significant amount of administered RBC, our results did not provide sufficient clinical evidence of the role of HH on decreased transfusion due to the relatively small sample size and different proportion of surgery types. Further studies with larger sample sizes are needed to investigate the incidence of postoperative complications.
Several previous studies have reported the effect of HH on the prevention of intraoperative hypothermia, but such studies included cases undergoing specialized surgical conditions such as liver transplantation [
15] or did not monitor esophageal temperature as an indication of T
C [
12,
13]. In contrast, in our present study, we monitored esophageal temperature, which is a well–known indicator of T
C [
1] and focused to the effect in elderly patients. Because T
C normally decreases more than 0.5°C during phase 1 [
1], the small difference in intraoperative T
C change between the two groups in the present study, which was less than 0.5°C, may be clinically important in terms of maintenance of intraoperative temperature homeostasis [
7,
8]. Elderly patients have decreased skeletal muscle mass, resulting in both lower resting metabolic rate [
26] and impaired metabolic response to cold stimulus [
27]. Moreover, even in the awakened state, T
C may not be maintained because of reduced vasoconstriction response to cold stress [
7]. Therefore, elderly patients are more vulnerable to intraoperative hypothermia than younger patients, and the effect of warming devices on maintaining T
C during general anesthesia of elderly patients can be important. Our results suggest the usefulness of HH for preventing intraoperative hypothermia.
There were possible limitations to this study of note. First, despite the significant difference in T
C between the two groups, the main outcome did not support our hypothesis. Thus, the results of our present study can be regarded as preliminary only and should be interpreted carefully. Intravenous administration of warmed fluid and application of PEEP [
28] may have contributed to the relatively small difference in T
C in the present study. Moreover, the number of cases of radical cystectomy, which involves intestinal manipulation, was different between the two groups, which may also contribute to the results. However, our study particularly focused on the phases of T
C decrease and suggested the role of HH in intraoperative temperature management. Second, although our positive result revealed the usefulness of HH in preventing T
C decrease, its effectiveness is still controversial and should not be overestimated. Previous studies showed that forced-air warming system [
19], as well as warmed intravenous fluid [
29] were more effective on intraoperative T
C decrease than HH. Therefore, HH should not be considered a novel device and may contribute, among other various warming techniques, to preventing intraoperative hypothermia.
In conclusion, HH is helpful in preventing TC decrease in elderly patients, particularly a phase 2 TC decrease. Although the present results indicate that HH use is effective during general anesthesia in elderly patients, the results should be interpreted prudently, and further studies with larger populations are needed to substantiate its use in clinical practice.
Go to :







 PDF
PDF Citation
Citation Print
Print


 XML Download
XML Download