Abstract
Hypoglossal nerve palsy after general anesthesia is an exceptionally rare complication, which has varied etiology. We present a case of unilateral hypoglossal nerve palsy resulting from repeated airway intervention for general anesthesia. A 57-year-old woman was scheduled to undergo modified radical mastectomy. During endotracheal intubation, the patient had Cormack’s grade III—a severe airway condition. After the first intubation attempt failed, the intubation was attempted a second time using a stylet inside the endotracheal tube with cricoid pressure; this attempt was successful. In the evening of the operation day, the patient complained of dysarthria and dysphagia. Physical examination revealed deviation of the tongue to the right, which may have been caused by traumatic hypoglossal nerve injury. This case reviews the pathophysiology, prevention, and management of hypoglossal nerve palsy.
The hypoglossal nerve is the 12th cranial nerve and is as a pure motor nerve. It innervates the muscles of the tongue and controls tongue movements required for speech, food manipulation, and swallowing. Hypoglossal nerve injury following endotracheal intubation under general anesthesia is a rare complication and can cause symptoms, such as dysarthria and dysphagia [1]. We present a case of unilateral hypoglossal nerve palsy after endotracheal intubation for general anesthesia with a severe airway condition.
A 57-year-old woman (weight, 71 kg; height, 163 cm) was scheduled to undergo modified radical mastectomy after diagnosis of invasive ductal carcinoma of the left breast. She had a medical history of abdominal hysterectomy with bilateral salpingo-oophorectomy for uterine leiomyoma 8 years ago and partial nephrectomy of the left kidney for renal cell carcinoma 7 years ago. Her body-mass index was 26.7, and her medical history revealed hypertension (American Society of Anesthesiologist Physical Status II).
The results of preoperative clinical and laboratory evaluations, including routine blood chemistry, simple chest radiography, and electrocardiogram were unremarkable. There were no remarkable findings in the preoperative airway evaluations (no apparent anatomical abnormalities, three-finger width mouth opening, three-finger width thyromental distance, and Mallampati grade II). No premedication was administered. On arrival at the operating room, as the patient’s pulse oximetry, electrocardiogram, non-invasive blood pressure, and bispectral index (BIS) were monitored. Preoxygenation was performed with 100% oxygen for approximately 5 min. General anesthesia was induced using the target controlled infusion (TCI) pump (Orchestra, Fresenius Vial, France) at the affected site with propofol 6 μg/ml and remifentanil 3 ng/ml. After verify that the patient was unconsciousness, vecuronium 8 mg was administrated for muscle relaxation, and mask ventilation was performed for about 3 min without difficulty. Endotracheal intubation with direct laryngoscopy (Macintosh 3, Heine, Germany) and a 7-mm plain endotracheal tube (Mallinckrodt, Covidien, USA) revealed a severe airway condition i.e., Cormack’s grade III (only the epiglottis was seen, glottis was not seen but the neck extension was normal). Intubation was performed by a highly competent anesthesiologist. The first intubation attempt was unsuccessful. After mask ventilation for 3 min, a J-shaped endotracheal tube with the stylet was inserted into the trachea at the second intubation attempt. During the second intubation attempt, cricoid pressure was applied to improve visualization of the vocal cord, but we could not visualize the vocal cord. The endotracheal tube was secured at 21 cm of the right mouth corner. The cuff of the endotracheal tube was inflated with air, and the pressure of the cuff was below 20 cmH2O. After intubation, we examined the oral soft tissue and teeth status; no injury was observed. No adjustment of the cuff volume was made during the operation. Total intravenous anesthesia using the TCI pump was maintained with propofol and remifentanil in air and oxygen.
The patient was placed at a 30° angle, elevated and in the supine position with slight leg elevation. The patient’s position was changed from supine to sitting several times during the surgery to compare the size and position of both the breasts. The surgery was uneventful, and anesthesia was induced for 6 h. The patient was extubated with any complications and monitored in a post-anesthesia care unit (PACU). There were no immediate postoperative complications.
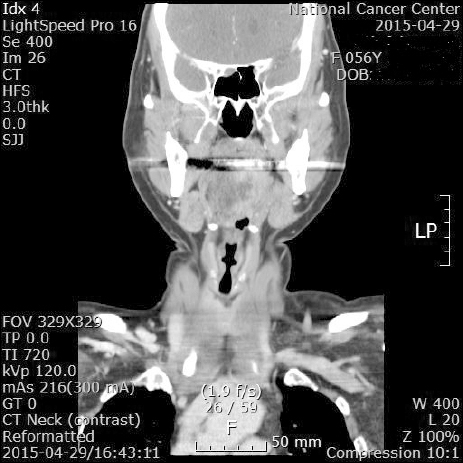
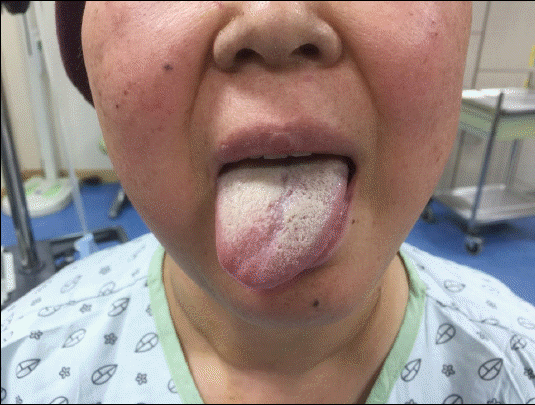
In the evening of the operation day, the patient complained of slurred speech and difficulty while swallowing and tongue movement. To exclude cerebrovascular diseases or internal carotid artery dissection, brain magnetic resonance imaging and neck computed tomography (CT) scan were performed as well as neurology and otorhinolaryngology consultations. No organic lesions were observed on the imaging studies. The base of the tongue showed interstitial swelling on the right side (Fig. 1). Physical examination revealed that the mobility of the tongue was deviated to the right side; however, no external lesion was observed (Fig. 2). The gag reflex and global and taste sensations of the tongue were normal. Symmetric uvula elevation and intact bilateral vocal fold movement were observed. There were no palpable mass lesions on the neck. These findings suggested isolated right hypoglossal nerve palsy. Oral prednisolone at a dosage of 70 mg/day was administered for a week, and the dosage was gradually reduced for the next 7 days. The patient was discharged on the postoperative day 18. On discharge, the patient still presented tongue deviation to the right side. However, the symptoms of dysarthria and dysphagia were improved. One month after discharge, the patient visited the otorhinolaryngology clinic. Physical examination showed that the tongue was still deviated to the right side.
The hypoglossal nerve arises from the hypoglossal nucleus of the caudal brain stem. After passing through the subarachnoid space, the nerve exits the skull-base of the posterior fossa through the hypoglossal canal. It spirals behind the vagus nerve and passes between the internal carotid artery and the internal jugular vein lying on the carotid sheath. After passing through the deep posterior belly of the diagastric muscles, it passes to the submandibular region, lateral to the hypoglossus muscle, and inferior to the lingual nerve to reach and innervate the tongue [2].
Taking into consideration the passage of the hypoglossal nerve as mentioned above, the nerve injury may be accompanied by other nerve injuries, such as injuries of the recurrent laryngeal nerve and lingual nerve. In case of accompanying injury of the recurrent laryngeal nerve with the hypoglossal nerve injury (Tapia’s syndrome), the patient may also show signs and symptoms of respiratory distress and hoarseness [3]. Similarly, in case of accompanying injury of the lingual nerve along with hypoglossal nerve injury, symptoms such as pain, loss of taste, and abnormal sensation of the tongue may be present. Therefore, thorough physical examinations are warranted to rule out accompanying nerve injuries of the recurrent laryngeal nerve and lingual nerve, when hypoglossal nerve injury is suspected.
Iatrogenic hypoglossal nerve injury following laryngeal mask airway (LMA) and endotracheal intubation has been described [4], but it is an extremely rare neurologic injury related to anesthesia care. The anatomical course of the 12th cranial nerve was located in a protective tissue with an exception of close proximity to the hyoid bone. Suspected mechanism of hypoglossal nerve injury related to intubation is excessive stretching of the nerve against the hyoid bone by the endotracheal tube itself or inflated cuff of the endotracheal tube. Direct compression by LMA, compression of the nerve by the posterior part of the laryngoscope, distension of the nerve by intubation with cricoid pressure, and compression of the nerve beneath the angle of the mandible by fingers during mask ventilation may also result in nerve injury [5]. Other causes of the hypoglossal nerve injury include malignancy, trauma, stroke, multiple sclerosis, Guillain-Barre syndrome, infection, and even hysteria [6]. Hypoglossal nerve palsy after general anesthesia in a patient without any underlying disease, anatomical variation, and surgical manipulation near the hypoglossal nerve region may be due to oropharyngeal airway manipulation.
In our case report, the patient has a severe airway condition and underwent repeated intubation attempts during anesthesia induction. With the exception of a severe airway condition and position change during the surgery, there were no other attributable causes for the nerve injury. No surgery was performed around the neck, no use of nitrous oxide gas, and no underlying disease to cause the nerve damage. In addition, the cuff pressure of the endotracheal tube was examined using a manometer during intubation to prevent excessive cuff pressure. Considering previously mentioned conditions, it is assumed that excessive mechanical force applied by the laryngoscope blade in the structure around the hypoglossal nerve during intubation may be a probable cause of nerve injury. During intubation, we inserted laryngoscopic blades at the right base of the tongue and then elevated the tongue by deviating the tongue slightly to the left, in case of a right-handed intubation performer, and therefore, pressure was not applied to the right side of the base of the tongue. Interstitial swelling at the base of the right side of the right tongue on a neck CT scan was consistent with this hypothesis. Excessive force applied by the laryngoscope blade may cause hematoma of the edematous lesion. Therefore, when a severe airway condition is predicted during intubation, anesthesiologists should take extreme care not to apply excessive mechanical force that may result in nerve injury and should consider using safer alternative modalities of intubation like video-laryngoscopy and light wands. Awake intubation with a fiberoptic bronchoscope could be considered; there is a case of a two-stage flexible fiberoptic bronchoscopic awake intubation in a severe airway patient [7]. Using these kinds of alternative intubation modalities may significantly reduce the possibility of hypoglossal nerve injury in a severe airway patient. Less experienced anesthesiologists should note this as they tend to apply more aggressive force during intubation [8].
In 1992, Mullins et al. [9] described hypoglossal neurapraxia following shoulder arthroscopy and repair of the rotator cuff with the patient in a beach chair position. The authors commented that unilateral hypoglossal nerve compression with a endotracheal tube had not been reported previously. They presumed that the reversible injury occurred during a change in position of the bed from 70 degrees to 30 degrees, causing the nerve to be compressed against the angle of the mandible. In our patient, we noted the position change from supine to sitting position. It is possible that the position change during the surgery could have caused some torsion or pressure on the neck, which may have resulted in stretching or compression injury to the nerve. However, the relatively short duration in the sitting position (less than 1–2 minutes) has little significance for nerve injury.
The clinical course of the hypoglossal nerve palsy after general anesthesia is generally self-limited and presents complete recovery within 6 months in most of cases; this kind of recovery pattern suggests that the nerve damage is a neurapraxic injury [4]. There is no definite treatment for hypoglossal nerve palsy. A short course of systemic steroids and vitamin B complex is widely administrated [1], and the short course of high dosage of intravenous prednisolone in combination with intramuscular poly-vitaminic therapy in Bell’s palsy has yielded good outcomes [10]. Additionally, supportive therapy like dietary modification, logopedic therapy, and electrical stimulation could be provided [11]. Many studies have recommended the use of systemic steroids in case of nerve damage with edema, but there are no controlled studies on the benefits of steroid therapy in a neurapraxic injury patient after surgery [11].
Electromyography (EMG) is important in case of muscle immobility for accurate diagnosis, management, and prognosis. EMG provides useful information for differential diagnosis during actual nerve injury or other possible causes of muscle immobility. It is also significant for prediction of the prognosis. In a previous case of vocal cord immobility, signs of recovery on EMG preceded clinical signs of recovery, which means EMG has prognostic efficacy [12]. However, we did not perform an EMG in this case. Thus, the prognosis of the injured nerve could not be predicted. However, despite the obvious benefits of the EMG, it is less informative in unwilling or uncooperative patients. It is also invasive, as it requires deep muscle penetration for achieving a clear EMG signal. Finally, EMG does not change the treatment strategy.
In conclusion, hypoglossal nerve palsy after general anesthesia is a rare complication and has many etiologies. Oropharyngeal airway manipulation is closely related with the occurrence of nerve damage in an otherwise healthy patient undergoing non-relevant surgery of the hypoglossal nerve. Anesthesiologists should ascertain the cause, prevention, and management of hypoglossal nerve palsy and should be extremely careful not to cause this kind of nerve injury when inducing general anesthesia.
REFERENCES
1. Hong SJ, Lee JY. Isolated unilateral paralysis of the hypoglossal nerve after transoral intubation for general anesthesia. Dysphagia. 2009; 24:354–6. DOI: 10.1007/s00455-008-9197-5. PMID: 18853225.

2. FitzGerald MJ, Gruener G, Mtui E. Clinical neuroanatomy and neuroscience. 2012. 6th ed. Philadelphia: Saunders/Elsevier;p. 216.
3. Cinar SO, Seven H, Cinar U, Turgut S. Isolated bilateral paralysis of the hypoglossal and recurrent laryngeal nerves (Bilateral Tapia’s syndrome) after transoral intubation for general anesthesia. Acta Anaesthesiol Scand. 2005; 49:98–9. DOI: 10.1111/j.1399-6576.2004.00553.x. PMID: 15675991.

4. Dziewas R, Lüdemann P. Hypoglossal nerve palsy as complication of oral intubation, bronchoscopy and use of the laryngeal mask airway. Eur Neurol. 2002; 47:239–43. DOI: 10.1159/000057906. PMID: 12037439.

5. Hung NK, Lee CH, Chan SM, Yeh CC, Cherng CH, Wong CS, et al. Transient unilateral hypoglossal nerve palsy after orotracheal intubation for general anesthesia. Acta Anaesthesiol Taiwan. 2009; 47:48–50. DOI: 10.1016/S1875-4597(09)60022-9.

6. Keane JR. Twelfth-nerve palsy. Analysis of 100 cases. Arch Neurol. 1996; 53:561–6. DOI: 10.1001/archneur.1996.00550060105023.
7. Chung MY, Kim CJ, Chea JS, Lee MN, Lee BH. The two stage flexible fiberoptic bronchoscoptic awake intubation in a patient with the symptomatic vallecular cyst: a case report. Anesth Pain Med. 2011; 6:146–9.
8. Garcia J, Coste A, Tavares W, Nuño N, Lachapelle K. Assessment of competency during orotracheal intubation in medical simulation. Br J Anaesth. 2015; 115:302–7. DOI: 10.1093/bja/aev207. PMID: 26170352.

9. Mullins RC, Drez D Jr, Cooper J. Hypoglossal nerve palsy after arthroscopy of the shoulder and open operation with the patient in the beach-chair position. A case report. J Bone Joint Surg Am. 1992; 74:137–9. PMID: 1734004.

10. Lagalla G, Logullo F, Di Bella P, Provinciali L, Ceravolo MG. Influence of early high-dose steroid treatment on Bell’s palsy evolution. Neurol Sci. 2002; 23:107–12. DOI: 10.1007/s100720200035. PMID: 12391494.
11. Shah AC, Barnes C, Spiekerman CF, Bollag LA. Hypoglossal nerve palsy after airway management for general anesthesia: an analysis of 69 patients. Anesth Analg. 2015; 120:105–20. DOI: 10.1213/ANE.0000000000000495. PMID: 25625257. PMCID: PMC4308816.
12. Guha K, Sabarigirish K, Singh SK, Yadav A. Role of laryngeal electromyography in predicting recovery after vocal fold paralysis. Indian J Otolaryangol Head Neck Surg. 2014; 66:394–7. DOI: 10.1007/s12070-014-0723-5. PMID: 26396950. PMCID: PMC4571471.





 PDF
PDF Citation
Citation Print
Print


 XML Download
XML Download