Abstract
Benedikt syndrome is characterized by ipsilateral ophthalmoplegia with contralateral hemichorea due to a midbrain lesion. A 67-year-old male with Benedikt syndrome underwent corpectomy at L1 and anterolateral interbody fusion at T12-L2 due to pathologic bursting fracture at L1 involving multiple myeloma. He had a history of traumatic subarachnoid hemorrhage and subdural hemorrhage 8 months before surgery. Magnetic resonance image of the brain revealed intracranial hemorrhage from thalamus to midbrain. Target controlled infusion with propofol and remifentanil were administered for anesthetic induction and maintenance and close hemodynamic and neurologic monitoring led to successful anesthetic management.
Go to : 
Benedikt syndrome is characterized by ipsilateral oculomotor nerve palsy and contralateral hemiparesis with involuntary movement caused by a midbrain lesion [1].
In 1889, Moritz Benedikt first described a patient with oculomotor paralysis and crossed hemiparesis with tremor. This rare syndrome is caused by infarction, hemorrhage, and a midbrain tumor, but a trauma origin is rare. Only one case of posttraumatic Benedikt syndrome has been reported in the English literature [1]. Due to the rarity of this condition, anesthetic management remains challenging because of the neurologic medications being taken, which might affect hemodynamics and anesthesia depth. In particular, hypotension and hypertension could aggravate the underlying brain lesion, and thus, strict hemodynamic control is essential. In a previous report of hemiballism-hemichorea, only 3 minutes of hypotension led to focal cerebral ischemia involving thalamus, pons, and midbrain [2].
No previous report has been issued on the anesthetic management of Benedikt syndrome in the English literature. Here, we describe general anesthetic management for spine fusion in a patient with mild hemiparesis with involuntary choreic movement and ptosis with diplopia after traumatic brain injury.
Go to : 
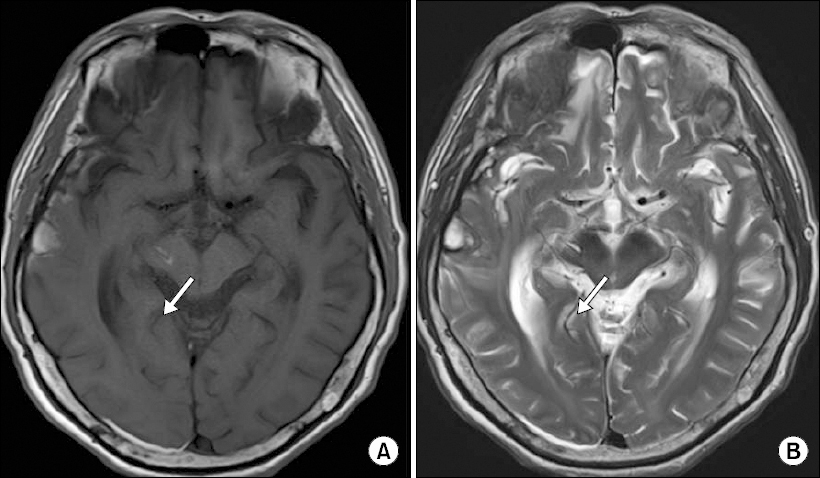
A 67-year-old male (height 163 cm, weight 56 kg) was scheduled for corpectomy at L1 and anterolateral interbody fusion at T12-L2 due to a pathologic bursting fracture at L1 attributed to multiple myeloma. He suffered from back pain and was diagnosed with lumbar spine involvement by multiple myeloma. Eight months before surgery, he experienced traumatic subarachnoid hemorrhage, subdural hemorrhage, and intracranial hemorrhage (Glasgow coma scale, 14) due to a fall down. During admission for traumatic brain injury, he complained of dysphagia, dysarthria, involuntary movement and tremor in the left arm and hand with grade 3 motor weakness. In addition, he suffered from right sided diplopia and ptosis. Brain magnetic resonance imaging showed right frontal base occipital hemorrhage with bilateral temporal hemorrhage, and intracranial hemorrhage on thalamus to midbrain (Fig. 1). His symptoms of involuntary upper limb movement and diplopia with ptosis persisted until admission for spine surgery. He was taking haloperidol (23 mg twice daily) and galantamine hydrobromide (16 mg twice daily). Preoperative laboratory findings revealed blood urea nitrogen (BUN)/creatinine 86/8.4 mg/dl, potassium 6.5 mEq/L, calcium 11.1 mg/dl, hematocrit 16%, and a platelet count of 138 × 103/mm.
He was diagnosed as having multiple myeloma involving kidney and spine, and received hemodialysis and 4 units of packed red blood cells to correct a hematocrit level of 28% immediately before surgery.
The patient was not premedicated. In the operating room, noninvasive blood pressure, bispectral index, pulse oximetry, and electrocardiogram monitors were applied. Initial vital signs were BP 149/81 mmHg and HR 73 beats/min. After local infiltration, a 20 G left radial arterial line was inserted and blood pressure was monitored continuously. Propofol 5 ug/ml and remifentanil 5 ng/ml (dosages were determined using the Schnider model and the Minto model, respectively) were administered using a target-controlled infusion device (Orchestra, Fresenius Vial, Brezins, France) for anesthesia induction. Propofol was adjusted to maintain an effect-site concentration of 2.0–3.5 ug/ml and bispectral index (BIS) values between 40 and 50, and remifentanil was adjusted to maintain an effect-site concentration of 0.5–3.0 ng/ml to maintain a mean arterial pressure within 20% of baseline. Rocuronium 50 mg was administered to facilitate tracheal intubation and no more rocuronium was used during the operation.
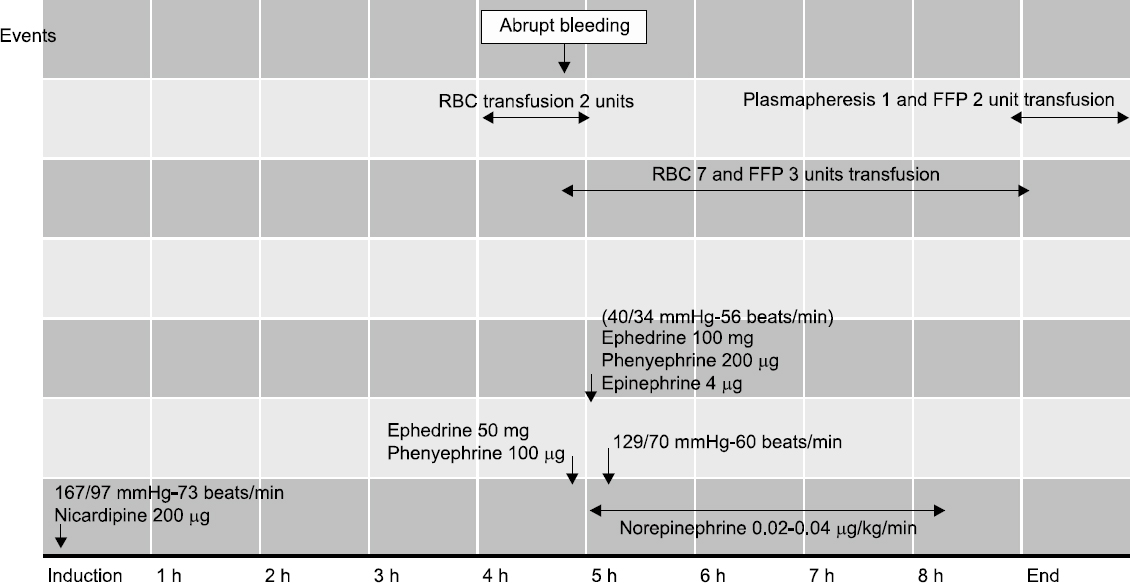
Mean arterial and central venous pressure via the right internal jugular vein were maintained at 75–85 mmHg and 7–10 mmHg using intermittent nicardipine, phenylephrine, and ephedrine administration (Fig. 2). At 4 hours 45 minutes after starting operation, blood pressure and central venous pressure dropped to 80/40 mmHg and 2 mmHg and restored within 1 min after injection of phenylephrine and ephedring. At 5 hours after starting operation, blood pressure and central venous pressure re-dropped to 40/34 mmHg and 0 mmHg after 2000 ml of abrupt surgical bleeding and ephedrine, phenylephrine and epinephrine were injected. And packed red blood cell were infused via rapid infusion system and restored the hemodynamic variables gradually and reached to 129/70 mmHg about 10 min after injecting vasopressors. End tidal CO2 was maintained between 33 and 40 mmHg and arterial blood gasses were analyzed (Table 1). Motor evoked potential and neuromuscular monitoring using train of four ratio were monitored during the 9-hour operation. Estimated blood loss was 3000 ml and 9 units of packed red blood cell and 4300 ml of infused fluid were transfused. Postoperatively, patient-controlled analgesia using fentanyl 800 ug in a total volume of 100 ml connected to an intravenous line was used for 48 hours, and palonosetron was administered to prevent postoperative nausea and vomiting.
After arrival in the surgical intensive care unit, his mean blood pressure was controlled at 75–85 mmHg. There were no adverse neurologic signs and no changes were detected by postoperative brain computed tomography. On postoperative day 3, the patient was transferred to a general ward. On postoperative day 37, velcade, melphalan, and prednisolone chemotherapy was started, and on postoperative day 46, sudden dyspnea and desaturation occurred due to aspiration ascribed to a pre-existing swallowing difficulty. He expired on postoperative day 56.
Go to : 
Our patient suffered from involuntary limb movement with mild hemiparesis and diplopia with ptosis after traumatic brain injury. For general anesthesia, we used a propofol and remifentanil target controlled infusion system. After considering the patient’s underlying neurologic condition and the drugs being taken, we considered close hemodynamic and neuromuscular monitoring could lead to successful anesthetic management. Furthermore, because the midbrain is critical for controlling hemodynamic response to blood loss, and hemorrhagic infarction commonly recurs within 2 years [3], distorted response to blood loss must be considered in cases of midbrain injury.
Benedikt syndrome has been called paramedian midbrain syndrome or red nucleus syndrome. The midbrain is near the cerebral cortex, cerebellum, and brain stem, and plays a significant role in visual and auditory processing and motor control [4]. Furthermore, a lesion involving tegmentum of the midbrain produces oculomotor nerve palsy, contralateral hemiparesis, and involuntary movement and is usually induced by infarction of posterior cerebral artery branches [1,4]. Bendikt syndrome is diagnosed based on clinical symptoms of oculomotor palsy and contralateral hemiparesis with involuntary movement and a midbrain involving lesion by imaging. Our patient experienced typical symptoms of Benedikt syndrome after traumatic brain injury involving midbrain. Recently, although deep brain stimulation had been reported to effectively reduce tremor in Benedikt syndrome [5], no confirmed treatment has been proposed.
Our patient was taking haloperidol and galantamine hydrobromide to relieve involuntary movement. Interestingly, Etezadi et al. [6] demonstrated that haloperidol reduced anesthetic requirements of propofol during intensive care unit sedation in agitated patients. Although we used a target controlled infusion system for propofol and remifentanil, there may have been discrepancy between effect site concentration and anesthetic level because of the effect of haloperidol, and thus, we monitored bispectral index to maintain anesthetic depth. It has been reported that both haloperidol and metoclopramide could aggravate involuntary movements by inducing an extrapyramidal side effect, and thus, we did not administered metoclopramide [7,8]. Five HT3 receptor antagonists are known to improve cognitive function and cerebella ataxia [9], and so palonosetron was used to prevent postoperative nausea and vomiting.
Galantamine hydrobromide (a cholinesterase inhibitor) could lead to inadequate response to non-depolarizing neuromuscular blocking agents, and therefore, neuromuscular function was monitored continuously throughout the operation. Furthermore, data regarding the relation between galantamine and motor evoked potential is scarce, but a previous clinical study demonstrated that motor evoked potential monitoring is reliable if T2/Tcontrol twitch height is > 0.5 or train of four count is > 2 [10]. Although it has also been reported that balanced desflurane anesthesia and total intravenous anesthesia provided similar hemodynamic responses and recovery profiles [11,12], total intravenous anesthesia is considered to have favorable pharmacokinetic and neurophysiological properties, to enhance the quality of intraoperative motor evoked potentials, and reduce the risk of procedure-related morbidity [13]. After considering the effects of inhalation agents on motor evoked potentials and train of four monitoring, we selected target controlled propofol and remifentanil infusion. Blood pressure was continuously monitored by invasive arterial blood pressure monitoring during and after operation and cardiovascular drugs were used judiciously. Both hyper- and hypotension can induce and aggravate central origin involuntary movement [4,14], and blood pressure maintenance within a tolerable range is an important aspect of anesthetic care. In this case, we experienced abrupt drops of blood pressure due to surgical bleeding. We prepared vasopressors and rapid infusion system before anesthetic induction and we immediately applied prepared vasopressors and rapid infusion system during surgical events and the patient did not suffered from neurologic complication after operation.
Another remarkable feature of this case was the presence of multiple myeloma. This hematologic malignancy increase susceptibility to stroke due to hyperviscosity, anemia, hypercalcemia, and renal failure. Proper fluid management could prevent hyperviscosity syndrome. Furthermore, altered plasma albumin to globulin ratio and renal impairments could change the pharmacokinetics of anesthetic drugs, and thus, close monitoring of hemodynamics and anesthesia depth are crucial for patients with multiple myeloma [15].
In conclusion, careful monitoring of hemodynamics and neuromuscular function and the selection of proper anesthetic drugs might improve prognosis in a patient with post-traumatic Benedikt syndrome.
Go to : 
REFERENCES
1. Paidakakos NA, Rokas E, Theodoropoulos S, Dimogerontas G, Konstantinidis E. Posttraumatic Benedikt’s syndrome: a rare entity with unclear anatomopathological correlations. World Neurosurg. 2012; 78:715e13–5. DOI: 10.1016/j.wneu.2012.03.028. PMID: 22484069.

2. Itoh H, Shibata K, Nitta E, Takamori M. Hemiballism-hemichorea from marked hypotension during spinal anesthesia. Acta Anaesthesiol Scand. 1998; 42:133–5. DOI: 10.1111/j.1399-6576.1998.tb05095.x. PMID: 9527738.
3. Bae H, Jeong D, Doh J, Lee K, Yun I, Byun B. Recurrence of bleeding in patients with hypertensive intracerebral hemorrhage. Cerebrovasc Dis. 1999; 9:102–8. DOI: 10.1159/000015906. PMID: 9973653.

4. Ruchalski K, Hathout GM. A medley of midbrain maladies: a brief review of midbrain anatomy and syndromology for radiologists. Radiol Res Pract. 2012; 2012:258–524. DOI: 10.1155/2012/258524.

5. Bandt SK, Anderson D, Biller J. Deep brain stimulation as an effective treatment option for post-midbrain infarction-related tremor as it presents with Benedikt syndrome. J Neurosurg. 2008; 109:635–9. DOI: 10.3171/JNS/2008/109/10/0635. PMID: 18826349.

6. Etezadi F, Najafi A, Yarandi KK, Moharari RS, Khajavi MR. ICU sedation with haloperidol-propofol infusion versus midazolam-propofol infusion after coronary artery bypass graft surgery: a prospective, double-blind randomized study. Ann Card Anaesth. 2012; 15:185–9. DOI: 10.4103/0971-9784.97974. PMID: 22772512.

7. Jo YY, Kim YB, Yang MR, Chang YJ. Extrapyramidal side effects after metoclopramide administration in a post-anesthesia care unit -A case report-. Korean J Anesthesiol. 2012; 63:274–6. DOI: 10.4097/kjae.2012.63.3.274. PMID: 23060988. PMCID: PMC3460160.

8. Moleman P, Schmitz PJ, Ladee GA. Extrapyramidal side effects and oral haloperidol: an analysis of explanatory patient and treatment characteristics. J Clin Psychiatry. 1982; 43:492–6. PMID: 7161250.
9. Ogawa M. Pharmacological treatments of cerebellar ataxia. Cerebellum. 2004; 3:107–11. DOI: 10.1080/147342204100032331. PMID: 15233578.

10. Kim WH, Lee JJ, Lee SM, Park MN, Park SK, Seo DW, et al. Comparison of motor-evoked potentials monitoring in response to transcranial electrical stimulation in subjects undergoing neurosurgery with partial vs no neuromuscular block. Br J Anaesth. 2013; 110:567–76. DOI: 10.1093/bja/aes395. PMID: 23378247.
11. Sloan TB, Toleikis JR, Toleikis SC, Koht A. Intraoperative neurophysiological monitoring during spine surgery with total intravenous anesthesia or balanced anesthesia with 3% desflurane. J Clin Monit Comput. 2015; 29:77–85. DOI: 10.1007/s10877-014-9571-9. PMID: 24643708.

12. Gokce BM, Ozkose Z, Tuncer B, Pampal K, Arslan D. Hemodynamic effects, recovery profiles, and costs of remifentanil-based anesthesia with propofol or desflurane for septorhinoplasty. Saudi Med J. 2007; 28:358–63. PMID: 17334459.
13. Scheufler KM, Zentner J. Total intravenous anesthesia for intraoperative monitoring of the motor pathways: an integral view combining clinical and experimental data. J Neurosurg. 2002; 96:571–9. DOI: 10.3171/jns.2002.96.3.0571. PMID: 11883843.

14. O’Riordan S, McGuigan C, Stevens J, Chapman N, Ball J. Reversible hypertensive cerebellar encephalopathy and hydrocephalus. J Neurol Neurosurg Psychiatry. 2007; 78:1008–9. DOI: 10.1136/jnnp.2006.107672. PMID: 17332051. PMCID: PMC2117878.
15. Prasad KK. Roizen M, Fleisher L, editors. Multiple myeloma. Essence of anaesthesia practice. 2002. 2nd ed. Philadelphia: WB Saunders Company;p. 229.
Go to : 




 PDF
PDF Citation
Citation Print
Print


 XML Download
XML Download