This article has been
cited by other articles in ScienceCentral.
Abstract
Background:
It is important to ensure that patients are normothermic during surgery. In total knee arthroplasty, the pneumatic tourniquet affects body temperature. We compared the ability of two warming devices to preserve core temperature in patients using a lower limb tourniquet under general anesthesia.
Methods:
We included 132 patients with American Society of Anesthesiologists physical status I–II who were scheduled to undergo total knee arthroplasty. The patients were randomly divided into four groups (n = 33): group 1, without any heating method; group 2, with fluid warming; group 3, with forced-air warming; and group 4, with a combination of the two heating methods. After the induction of anesthesia, the esophageal and urinary bladder temperatures were monitored and recorded every 5 min before tourniquet deflation and every 1 min after tourniquet deflation.
Results:
Before tourniquet deflation, compared with group 1, the odds ratios of groups 3 and 4 were less than 1. After tourniquet deflation, compared with group 1, the odds ratios of all groups using warming devices were less than 1. In particular, group 4 showed the largest hypothermia-preventive effect among the four groups. There was a significant correlation between esophageal temperature and bladder temperature before and after tourniquet deflation.
Conclusions:
After tourniquet deflation, a combination of a fluid warmer and forced-air warmer is the most effective method to prevent hypothermia, although either a fluid warmer or forced-air warmer alone could help to prevent hypothermia. Urinary bladder temperature changes correlate well with esophageal temperature changes throughout this operation.
Keywords: Bladder temperature, Esophageal temperature, Total knee replacement, Tourniquet
INTRODUCTION
The pneumatic tourniquet was introduced in 1904 by Harvey Cushing to decrease bleeding and ensure the cleanliness of the operative field in limb surgery. It is still commonly used. However, this procedure may lead to intraoperative hemodynamic changes and may induce metabolic changes in the limb where the pneumatic tourniquet is applied, such as decreases in pH and PO
2 and increases in PCO
2, K
+, and lactate [
1]. Moreover, inflation and deflation of the pneumatic tourniquet, in conjunction with anesthesia, causes changes in the core temperature [
2-
4]. When the tourniquet is inflated, the blood pressure and heart rate increase, leading to an increase in body metabolism and heat production. However, because the heat cannot be redistributed to the distal part of the limb beyond the tourniquet, the core temperature increases [
1,
5]. Tourniquet inflation also interrupts perfusion of the distal part of the limb, resulting in an ischemic state. Consequently, energy sources for cellular metabolism become deficient and the temperature of the distal part of the limb decreases through exposure to the temperature of the room. After the tourniquet is deflated, warmer blood from the body core starts to perfuse to the distal part beyond the tourniquet, and cooler blood from the distal part of the limb flows into the systemic circulation, causing the core temperature to decrease [
4-
6]. Mild perioperative hypothermia can even lead to more serious complications, such as wound infection, prolongation of postoperative care unit and hospital stays, severe cardiac complications and ventricular tachycardia, coagulopathy, shivering, and reduced immune function. Therefore, continuous monitoring of the core body temperature and careful management of the patient are required [
7-
11].
To prevent hypothermia, many anesthesiologists use tools such as forced-air warmers, fluid warmers, and respiratory gas humidifiers. Of these devices, forced-air warmers and fluid warmers are considered the most effective [
12-
14]. The aim of our present study was to compare the hypothermia-preventive effects of forced-air and fluid warmers in patients undergoing unilateral total knee replacement arthroplasty under general anesthesia in whom a rapid decrease in core body temperature is expected after tourniquet deflation. In addition, the effect of the combination of the two devices was evaluated. The relationship between esophageal temperature and urinary bladder temperature during tourniquet deflation was also analyzed.
MATERIALS AND METHODS
This was a randomized single-blind study of patients who were treated from September 2011 to February 2012 at our hospital. We enrolled patients who were older than 50 years and undergoing unilateral total knee replacement arthroplasty under general anesthesia with an American Society of Anesthesiologists physical status I or II. Patient exclusion criteria were lack of informed consent; use of medication that could affect body temperature, such as anticholinergic agents; presence of a disease that could influence body temperature, including central nervous system diseases, thyroid diseases, malignant hyperthermia, or infections; receipt of treatments such as transfusion that could affect body temperature; a highly obese body weight (body mass index [BMI] > 35 kg/m2); and a planned bilateral total knee replacement arthroplasty. After providing written informed consent, 132 consecutive patients were enrolled.
The included patients were divided into four groups; group 1, the control group without any warming device; group 2, treated with a fluid warmer (Thermosens CE 0120; Sewoon Medical Co., Cheonan, Korea); group 3, treated with a forced-air warmer (3M™ Bair Hugger™ Warming Unit, Model 505; Arizant Health Care Inc., Minneapolis-St. Paul, MN, USA); and group 4, treated with both types of warmers. In order to minimize the possibility of a selection bias and obtain a high level of objectivity, patients were allocated to the groups by randomization. A randomization sequence was created by SAS version 8.2 (SAS Institute Inc., Cary, NC, USA) prior to beginning the clinical trial.
No patients were premedicated prior to anesthesia. When patients arrived at the operating room (OR), standard patient monitoring was performed, including blood pressure, continuous electrocardiographic data, heart rate, oxygen saturation via pulse oximetry, and end-tidal CO2. Anesthesia was induced with 4–5 mg/kg of thiopental sodium and 0.6–0.9 mg/kg of rocuronium as a muscle relaxant and was maintained with sevoflurane in a mixture of O2 and N2O. Ventilation was mechanically controlled with an inspired oxygen fraction of 50%. Anesthetic gases were delivered using a fresh gas flow of 2 L/min and were not actively humidified or heated. The end-tidal concentration of sevoflurane was controlled between 2.0 and 3.0 volume percent with regard to the changes in the blood pressure and heart rate. The minute ventilation was adjusted to maintain an end-tidal CO2 partial pressure between 30 and 33 mmHg.
During the operation, lactated Ringer’s solution was infused by considering the compensatory intravascular volume expansion caused by anesthesia, deficits, losses, and maintenance according to the “4-2-1 rule” [
15]. Heart rate, blood pressure, electrocardiography, oxygen saturation, and the core body temperature were continuously monitored. When the systolic blood pressure decreased below 20% of the initial value or dropped below 80 mmHg, 10 mg of ephedrine or 100g of phenylephrine was injected, depending on the heart rate value.
The ambient temperature of the OR was measured at a height of 2 m using a mercury thermometer attached to the wall and was maintained between 22°C and 24°C. For the patients in group 2, fluid warmed to 38°C was infused through a 1 m length tube under a blanket to maintain the patient’s temperature after entering the OR. For the patients in group 3, a forced-air warmer set on high (38°C) was applied immediately to the patient after entering the OR. The hose from the forced-air warmer was surrounded by a blanket and placed on the patient’s trunk above the level of the femur. For the patients in group 4, a fluid warmer and a forced-air warmer were applied in the same manner as in groups 2 and 3, respectively.
After anesthesia induction, temperature probes were inserted into the esophagus and bladder of all patients. No additional warming devices were used other than the assigned methods described above. If a patient’s core body temperature dropped below 35°C, an additional warming method was applied. The tourniquet was inflated by 100–150 mmHg higher than systolic blood pressure measured at the upper limb. Sevoflurane was maintained at 2.0–3.0 volume percent during anesthesia and was discontinued at the end of surgery.
Blood pressure, heart rate, oxygen saturation, and end-tidal CO2 were continuously monitored. The core body temperature was measured through an esophageal temperature probe (esophageal stethoscope; DeRoyal Industries Inc., Powell, TN, USA) located at the distal 1/3 portion of the esophagus. The esophageal temperature was recorded at 5 min intervals before tourniquet deflation and at 1 min intervals after tourniquet deflation. Bladder temperature, representative of intermediate temperature, was measured and recorded at the same time interval using a bladder temperature probe (Foley catheter with temperature sensor; DeRoyal Industries Inc.).
All general characteristics are presented as the mean ± standard deviation (SD). Statistical analysis was performed using SPSS 12.0 (SPSS Inc., Chicago, IL, USA). The demographic characteristics of each group were compared using one-way ANOVA. In our present study, hypothermia was defined as a core body temperature below 36°C. Logistic regression with binominal distribution was used to compare the ratio of hypothermia out of all the body temperatures measured in each group. The analysis was processed before and after tourniquet deflation. Univariate analysis was first performed and then multivariate logistic regression was carried out using relevant patient demographics.
The difference between the simultaneously recorded esophageal and bladder temperatures was analyzed by the Pearson’s correlation test. Analysis was also subdivided into two periods, before and after tourniquet deflation. A P value smaller than 0.05 was considered statistically significant.
RESULTS
A total of 132 patients (122 women, 10 men) were enrolled in this study. These individuals were scheduled to receive a unilateral total knee arthroplasty with a tourniquet under general anesthesia. Thirty-three patients were allocated to each of the four groups. The general characteristics of each group are listed in
Table 1 and the changes in the mean temperature measured at the esophagus with time are shown in
Fig. 1.
Table 1
General Characteristics of the Study Patients
|
Group 1 (n = 33) |
Group 2 (n = 33) |
Group 3 (n = 33) |
Group 4 (n = 33) |
P value |
|
|
|
|
|
Mean |
SD |
Mean |
SD |
Mean |
SD |
Mean |
SD |
|
Age (yr) |
68.7 |
6.8 |
68.2 |
5.7 |
69.9 |
6.9 |
69.2 |
6.4 |
0.3438 |
|
BMI (kg/m2) |
27.26 |
3.41 |
27.80 |
2.89 |
27.80 |
3.56 |
25.28 |
3.13 |
0.0014*
|
|
Number of male (%) |
0 (0.0%) |
3 (9.1%) |
3 (9.1%) |
5 (15.2%) |
0.1441 |
|
Total urine output |
141.21 |
97.80 |
113.94 |
118.21 |
125.21 |
97.79 |
143.79 |
159.87 |
0.1938 |
|
Crystalloid infused (ml) |
760.61 |
283.05 |
591.52 |
206.46 |
719.70 |
306.17 |
654.55 |
214.83 |
0.0494*
|
|
Colloid infused (ml) |
620.00 |
203.67 |
659.09 |
149.72 |
573.44 |
257.78 |
625.76 |
244.99 |
0.3867 |
|
Tourniquet time (min) |
96.64 |
24.15 |
107.18 |
21.71 |
103.97 |
20.61 |
106.42 |
26.22 |
0.1295 |
|
Anesthetic time (min) |
155.00 |
20.12 |
163.27 |
18.65 |
159.09 |
21.22 |
156.97 |
16.91 |
0.2641 |
Fig. 1
Mean esophageal temperature of each group throughout the procedure under general anesthesia. Group 1, control group without any warming devices; group 2, the fluid-warmed group; group 3, the forced-air–warmed group; group 4, warmed using both warming devices. Itime, time elapsed from anesthesia induction; Dtime, time elapsed from tourniquet deflation.
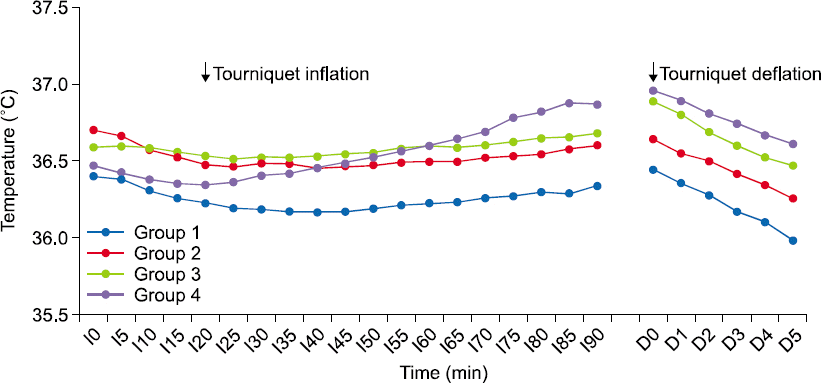
All groups showed an initial core body temperature decline under general anesthesia and the average body temperature began to increase 20 min after anesthesia in the groups treated using the warming devices. The average body temperatures of all groups declined after tourniquet deflation, but no patient showed a temperature lower than 35°C during the whole study period. Moreover, addition of a warming method other than the scheduled device was not required in any patient.
Univariate analysis for the “before tourniquet deflation” period showed that use of warming devices had a significant effect on preventing hypothermia (overall P value < 0.0001). Compared with group 1, the odds ratio of groups 2, 3, and 4 were 0.535 (P < 0.0001), 0.364 (P < 0.0001), and 0.572 (P < 0.0001), respectively. In other words, all of the warming devices effectively maintained body temperature before tourniquet deflation. When group 2 was indicated as the reference, group 3 had a significantly lower odds ratio (0.679 [P = 0.0253]), but group 4 did not (1.067 [P = 0.6799]). As for the comparison between groups 3 and 4, hypothermia prevention was significantly more effective in group 3 than in group 4. The results from the multivariate analysis adjusted for the variables in
Table 1 except for sex for the “before tourniquet deflation” period showed two differences from the univariate analysis. First, hypothermia prevention was not significant in group 2. Second, hypothermia prevention was significantly better in group 4 compared with group 2 (
Table 2).
Table 2
Logistic Regression for Hypothermia before Tourniquet Deflation Univariate Analysis (Overall P value <0.0001)
|
Group 1 |
Group 2 |
Group 3 |
Group 4 |
|
OR |
Ref |
0.535 (0.403–0.711) |
<0.0001 |
0.364 (0.265–0.499) |
<0.0001 |
0.572 (0.431–0.758) |
0.0001 |
|
|
Ref |
0.679 (0.484–0.953) |
0.0253 |
1.067 (0.784–1.453) |
0.6799 |
|
|
|
|
Ref |
1.571 (1.121–2.202) |
0.0088 |
|
|
Multivariable Analysis* (Overall P value <0.0001). |
|
|
Group 1
|
Group 2
|
Group 3
|
Group 4
|
|
|
OR |
Ref |
0.893 (0.642–1.243) |
0.5024 |
0.317 (0.221–0.455) |
<0.0001 |
0.558 (0.398–0.781) |
0.0007 |
|
|
Ref |
0.355 (0.243–0.517) |
<0.0001 |
0.624 (0.422–0.924) |
0.0184 |
|
|
|
|
Ref |
1.759 (1.148–2.697) |
0.0096 |
Univariate analysis for the “after tourniquet deflation” period showed that there were significant differences among the warming device methods (overall P value < 0.0001). Compared with group 1, the odds ratios of all warming groups were significantly lower (group 2, 0.454 [P < 0.0001]; group 3, 0.239 [P < 0.0001]; group 4, 0.143 [P < 0.0001]). When groups 3 and 4 were compared with group 2 as the reference, both showed hypothermia-preventive effects (group 3, 0.526 [P < 0.0001]; group 4, 0.315 [P < 0.0001]). Group 4 was more effective in maintaining normothermia than group 3. The results from the multivariate analysis adjusted for “before tourniquet deflation” information and the variables in
Table 1—except for sex, BMI, tourniquet time, and total urine output—were similar to those of the univariate analysis (
Table 3).
Table 3
Logistic Regression for Hypothermia after Tourniquet Deflation Univariate Analysis (Overall P value <0.0001)
|
Group 1 |
Group 2 |
Group 3 |
Group 4 |
|
OR |
Ref |
0.454 (0.357–0.577) |
<0.0001 |
0.239 (0.185–0.308) |
<0.0001 |
0.143 (0.108–0.189) |
<0.0001 |
|
|
Ref |
0.526 (0.404–0.685) |
<0.0001 |
0.315 (0.237–0.418) |
<0.0001 |
|
|
|
|
Ref |
0.598 (0.444–0.806) |
0.0007 |
|
|
Multivariable Analysis* (Overall P value <0.0001). |
|
|
Group 1
|
Group 2
|
Group 3
|
Group 4
|
|
|
OR |
Ref |
0.522 (0.378–0.719) |
<0.0001 |
0.367 (0.265–0.507) |
<0.0001 |
0.129 (0.089–0.189) |
<0.0001 |
|
|
Ref |
0.703 (0.504–0.981) |
0.0381 |
0.248 (0.169–0.364) |
<0.0001 |
|
|
|
|
Ref |
0.353 (0.236–0.527) |
<0.0001 |
The Pearson’s correlation coefficient was used to analyze the potential correlation between bladder and esophageal temperatures. During tourniquet inflation, this coefficient displayed a highly statistically significant association (r = 0.791,
Fig. 2). During tourniquet deflation, the coefficient was also considered to indicate a highly statistically significant association (r = 0.909,
Fig. 3).
Fig. 2
Pearson’s correlation between esophageal temperature and bladder temperature before tourniquet deflation. The Pearson’s correlation coefficient was calculated as 0.791.
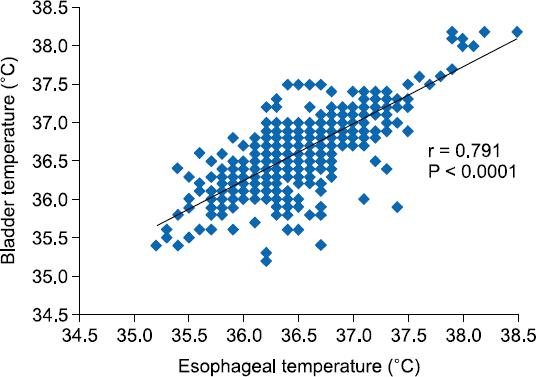
Fig. 3
Pearson’s correlation between esophageal temperature and bladder temperature after tourniquet deflation. The Pearson’s correlation coefficient was calculated as 0.909.

DISCUSSION
In our current study, a body core temperature below 36°C was defined as hypothermia. In order to compare the ability of two warming devices to prevent hypothermia during total knee arthroplasty under general anesthesia, we compared the ratio of hypothermia based on the esophageal temperature among the control, fluid-warmed, and forced-air–warmed groups, and a combination of the two warming device groups.
Sessler [
5] described a 1°C–1.5°C drop occurring in body temperature within 1 h after induction of general anesthesia to be the result of vasodilation. Vasodilation causes heat loss and the heat from the central body core is subsequently redistributed to the peripheral body. The results of the present study are in agreement with those of previous studies, namely, that body temperature decreases under general anesthesia, because the average body temperature continuously decreased until 40 min after general anesthesia induction in the control group. In contrast, the average body temperature of the experimental groups began to increase 30 min after the induction of anesthesia due to the effect of the warming devices and tourniquet inflation.
The previous study by Kim et al. [
16] showed that forced-air warmers can effectively maintain a normothermic core body temperature during unilateral total knee arthroplasty under general anesthesia. After deflation of the tourniquet, the forced-air warmer group showed a lower body temperature reduction than the group not using intraoperative warming devices. In our present study, we further compared fluid warming with forced-air warming and tested the potential advantage of using a combination of warming devices. Our findings were consistent with those of the previous study of Kim and colleagues because we also found that forced-air warming could effectively maintain normothermia after tourniquet deflation. In addition, we found that a combination of fluid warming and forced-air warming provided further benefit and that fluid warming alone was sufficient for preserving body temperature after tourniquet deflation.
A remarkable outcome of our present analyses was that the results depended on the tourniquet inflation phase (before vs. after). Before pneumatic tourniquet deflation, forced-air warming and a combination of forced-air and fluid warming effectively prevented hypothermia and forced-air warming alone was more effective than the combination. On the other hand, after tourniquet deflation, all warming devices effectively maintained patients in the normothermic range and their combination was the best method for preventing hypothermia. These results may be explained by differences in the flow rate of fluid. Horowitz et al. [
17] reported that the “Ranger”, which is a type of dry heat blood/fluid warmer similar to the “Thermosens”, has no clinical effect on heat transfer at a flow of 1 L/h. The low flow rate allows cooling of the fluid after it exits the warming unit; a high flow rate makes heat delivery possible. There is no need to infuse fluid rapidly during the tourniquet inflated period when bleeding is limited and vital signs are stable. In our current study, the flow rate of fluid infusion was also lower than 1 L/h before tourniquet deflation. After tourniquet deflation, however, the blood pressure tends to decrease and the flow rate of fluid infusion is relatively high. This dynamic gives rise to the differences between the two tourniquet phases.
We also investigated the ability of a bladder thermometer to measure core body temperature during total knee arthroplasty. All organs produce heat, but at different rates. Generally, the venous blood temperature reflects the mean organ temperature. Therefore, pulmonary artery catheters are the most accurate way to measure core temperature [
17]. However, this approach is invasive, so the core temperature is usually measured at the distal esophagus, tympanic membrane, or larynx [
18]. An esophageal thermometer is economical and accurate but difficult to use with monitored anesthesia care (MAC) or after surgery. In contrast, a bladder thermometer can be used with MAC or after surgery, but the accuracy varies according to urine output [
14]. The results obtained using a bladder thermometer are different from those of an esophageal thermometer, but there was a strong correlation between the two locations at all intervals measured. Cork et al. [
19] suggest that bladder temperature is as accurate a source for core temperature measurement as the esophagus, rectum, larynx, or the tympanic membrane. Lefrant et al. [
20] have shown that the temperature of the pulmonary artery is consistent with the temperature measured in the bladder and esophagus, but not at the rectum, axilla, or inguinal area. In our current study, the esophageal temperature was significantly correlated with the bladder temperature. Hence, a bladder thermometer can accurately and efficiently measure core body temperature. However, Fallis [
21] suggested that the bladder temperature does not reflect rapid changes in the core temperature. Additionally, Sato et al. [
22] determined that bladder temperature is accurate only when a high urine volume is present during noncardiac surgery. This should be taken into account when using a bladder thermometer to measure core body temperature.
Our study design had some limitations that require discussion. First, the ambient temperature of the OR was controlled from 22°C to 24°C. The room temperature affects thermal radiation, convection from the skin, and the degree of metabolic heat loss by evaporation from the skin incision [
23]. El-Gamal et al. [
24] suggest that it is beneficial to keep the ambient temperature at 26°C to prevent perioperative hypothermia during general anesthesia. Morris [
25] have shown that the ambient temperature of the OR is related to hypothermia. When surgery is performed in a 21–24°C room, 30% of the group becomes hypothermic, but no patients become hypothermic when the surgery is performed at 24–26°C. However, the OR temperature may influence the polymerization of bone cement and surgeons usually wear many layers of clothing and work under stressful conditions, so they are highly sensitive to the ambient temperature of the OR [
14,
26]. We thus ensured that the OR temperature was relatively low and, although the body temperature of the experimental groups were higher than those of the control group before tourniquet deflation, the body temperature of the control group was also maintained relatively well near the baseline until tourniquet deflation.
Second, there were a few significant differences in the general characteristics of the patients among the groups. The BMI of group 4 was lower than that of the other three groups. Fernandes et al. [
27] reported that the average core body temperature is maintained during the perioperative period at a higher level with air warmers in obese patients than in normal patients. Not only does thermal conduction occur more slowly in obese patients, but also the heat is produced to a greater degree by sympathetic nervous system activation through leptin secreted from fat cells [
28,
29]. However, as the body temperature of the combined warmed group was better maintained than that of other groups, this may have had a minor effect on the study results. The other general characteristic that was significantly different among the groups was the total amount of crystalloid infused throughout the anesthesia. The amount of crystalloid infused of group 2 was significantly lower than that of other groups. The total amount of crystalloid infused may be correlated with flow rate, so it may affect the results, as mentioned above.
Third, the use of phenylephrine may have affected the results. Ikeda et al. [
30] studied how phenylephrine infusion affects the core body temperature under general anesthesia. In that study, phenylephrine infusion reduced the magnitude of redistribution hypothermia. However, no patient needed phenylephrine injection to maintain a stable blood pressure in our current study series. In addition, it is considered that a single bolus injection of phenylephrine has no important effect on the redistribution of heat.
Fourth, no analysis of postoperative complications was performed in our present study. The major rationale for preventing perioperative hypothermia is to reduce complications and to improve outcomes [
7-
11]. Further analysis of the frequency of complications, including shivering, may have shown the actual benefit of the warming devices in clinical outcomes.
In conclusion, the use of fluid warmer or forced-air warmer devices effectively preserves the core body temperature after tourniquet deflation. The combination of the two warming devices leads to better normothermia preservation than use of a fluid warmer or forced-air warmer alone. Urinary bladder temperature changes strongly correlate with esophageal temperature changes throughout the operation, indicating that this noninvasive monitoring site may be useful.





 PDF
PDF Citation
Citation Print
Print




 XML Download
XML Download