A 48-year-old male was admitted to our hospital in July 2009 due to upper abdominal pain, nausea, vomiting, and weight loss lasting 4 weeks. The patient had no personal or family medical history of a malignant neoplasm. Gastroscopy revealed an antropyloric neoformation, 3 cm in diameter, and biopsy of the gastric lesion was positive for gastric SRC carcinoma. Computed tomography scan confirmed the gastric tumor and showed a coexistent massive mesenteric abdominal mass, with enlarged para-aortic, aorto-caval, and coeliac axis lymph nodes. A subtotal gastrectomy with D2 lympho-adenectomy and an excisional mesenteric node biopsy were performed. Histologic examination was consistent with two synchronous malignancies: a poorly differentiated intramucosal gastric SRC adenocarcinoma with embolic micrometastases in 2+/19 nodes of the stomach greater curvature (pT1, N1, M0, stage IB), and a follicular NHL (FL), grade 3a (> 15 centroblasts/high-power field and centrocytes present in the sample). Immunohistochemical staining of B FL cells revealed the co-expression of CD20, BCL6, BCL2, and CD79a within the B neoplastic follicles and a Ki-67 index > 20%. Bone marrow biopsy showed sporadic interstitial aggregates of small lymphoid CD20 and CD3 positive elements. After surgery, the patient showed a good recovery and was discharged on postoperative day 9. The surgical procedure was considered appropriate for early-stage I-B gastric cancer so no adjuvant chemotherapy was administered. However, a systemic chemotherapeutic regimen was selected for the FL bulky disease. The patient received seven cycles of a chemotherapy regimen including, on day 1: cyclophosphamide 750 mg/m
2, doxorubicin 50 mg/m
2, and vincristine 1.4 mg/m
2, and on days 1-5: prednisone 100 mg (CHOP regimen).The treatment was well-tolerated and induced a complete response. Two years later, a positron emission tomography (PET) scan showed disease recurrence, with mesenteric lymph nodes enlargement and increased
18F-fluorodeoxyglucose uptake (maximum standardized uptake value, 7) (
Fig. 1). The patient was treated with eight cycles of R-CNOP (day 1: RTX 375 mg/m
2; day 2: cyclophosphamide 750 mg/m
2, mitoxantrone 10 mg/m
2, vincristine 2 mg; days 2-6: prednisone 100 mg). He achieved complete remission and in February 2013 he began maintenance therapy with RTX (MR), at a dose of 375 mg/m
2 every 3 months. After eight MR cycles the patient suffered a 2-month period of watery diarrhea with a frequency of 3-4 times a day, of mushy stool that occasionally contained mucus, together with periumbilical and right abdominal pain. A surveillance PET scan, performed at that time, showed an increased activity in the terminal ilium (TI) and mesenteric lymphadenopathy. An ileo-colonoscopy revealed no significant abnormality in the colon mucosa, but macroscopic inflammatory changes in the TI including an erythematous mucosa and aphthous erosions covered with fibrin. Biopsy demonstrated active nonspecific ileitis. Treatment with 5-aminosalicylates (5-ASA) induced a prompt relief of symptoms. He was treated with another six cycles of RTX for a presumed recurrence of the lymphoma. A follow-up computed tomography enterography, performed 6 months later, showed resolution of the mesenteric adenopathy but the presence of a modest hyperenhanced bowel wall thickening in the terminal ileum. In September 2017, two months after the last cycle of RTX, the patient’s clinical conditions worsened. He developed bloody diarrhea, cramping abdominal pain, anemia and weight loss. Endoscopic evaluation showed a transmural involvement of the TI by an inflammatory process, with mucosal damage, deep ulceration, and edema. Histological examination revealed goblet cell depletion and active-chronic inflammation with crypt abscess formation (
Fig. 2A); the lamina propria was occupied by granulation tissue with dilated, inflamed capillaries. Immunohistochemistry analysis showed a total depletion of the CD20 positive B cells in the ileal mucosa (
Fig. 2B), an increased cellularity of CD3
+ T lymphocytes in the tonaca propria and intraepithelial mucosa (
Fig. 2C) and moderate excess of enlarged macrophages (CD68
+), exclusively in the lamina propria (
Fig. 2D), suggesting exacerbation of the Crohn’s disease. Pathological features were in keeping with active Crohn’s disease.
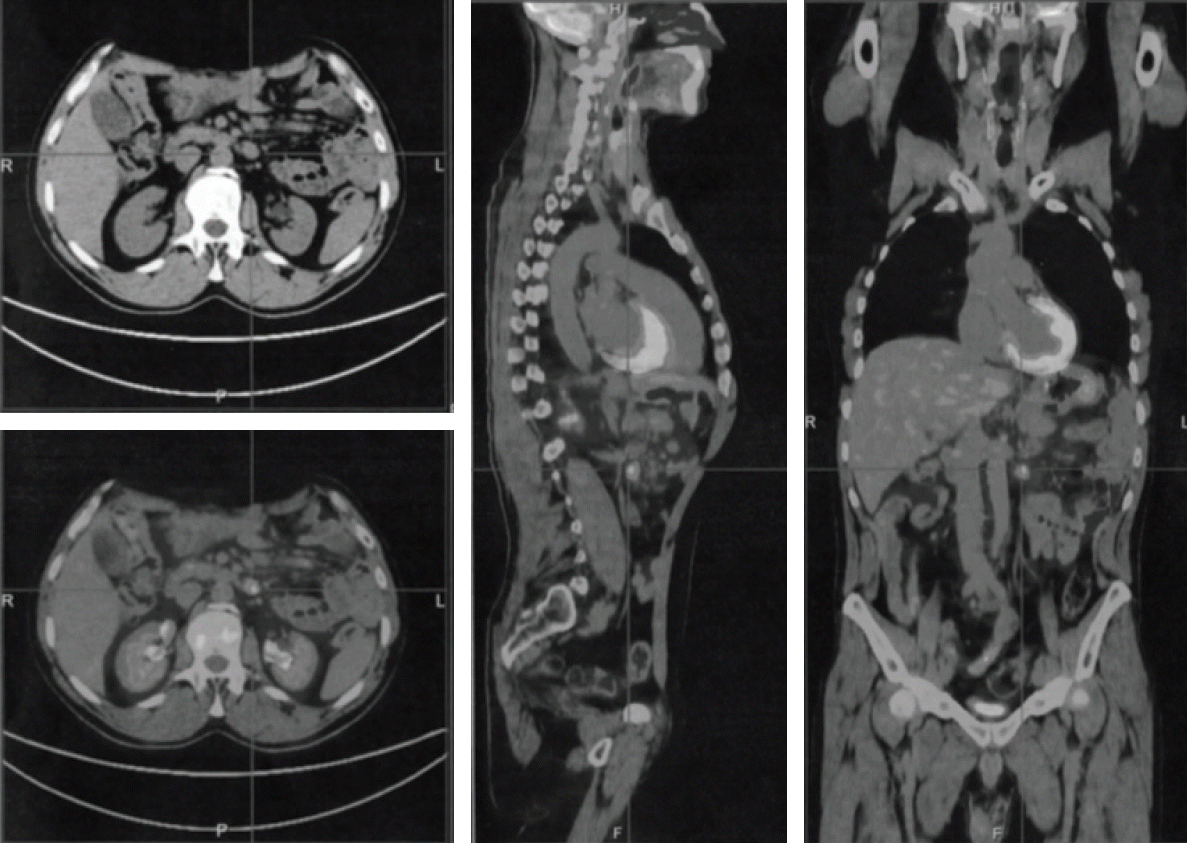 | Fig. 1.Positron emission tomography scan showing massive 18F-fluorodeoxyglucose uptake (maximum standardized uptake value, 7) and mesenteric lymph nodes enlargement. 
|
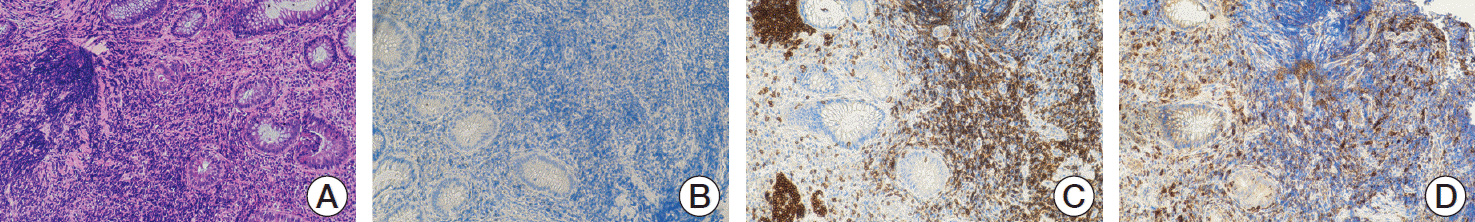 | Fig. 2.(A) Active-chronic inflammation with ulceration, crypt abscess formation and goblet cell depletion (H&E staining, ×5), (B) After rituximab therapy, CD20 cells staining was negative. (C) Immunohistochemistry with an anti-CD3 antibody demonstrated numerous intraepithelial mucosal T cells after therapy. (D) High tonaca propria and intraepithelial infiltration of macrophages (CD68+) (B-D, ×10). 
|
RTX therapy was interrupted. He was treated with budesonide and 5-ASA, and responded well. After 10 weeks the patient was asymptomatic and an endoscopic control showed slight signs of inflammation of the TI. A follow-up PET scan was negative for activity in the TI. He has remained in remission for 30 months without any adverse events. At present he is taking 5-ASA as maintenance therapy and the clinical conditions have clearly improved.
Written informed consent was obtained from the patient prior to publication of this case report and all procedures performed were in accordance with the ethical standards of the institutional research committee (IRCCS Giovanni Paolo II, Bari, Italy).






 PDF
PDF Citation
Citation Print
Print


 XML Download
XML Download