Abstract
Purpose
The systemic inflammation response index (SIRI) has been reported to have prognostic ability in various solid tumors but has not been studied in gallbladder cancer (GBC). We aimed to determine its prognostic value in GBC.
Materials and Methods
From 2003 to 2017, patients with confirmed GBC were recruited. To determine the SIRI’s optimal cutoff value, a time-dependent receiver operating characteristic curve was applied. Univariate and multivariate Cox analyses were performed for the recognition of significant factors. Then the cohort was randomly divided into the training and the validation set. A nomogram was constructed using the SIRI and other selected indicators in the training set, and compared with the TNM staging system. C-index, calibration plots, and decision curve analysis were performed to assess the nomogram’s clinical utility.
Results
One hundred twenty-four patients were included. The SIRI’s optimal cutoff value divided patients into high (≥ 0.89) and low SIRI (< 0.89) groups. Kaplan-Meier curves according to SIRI levels were significantly different (p < 0.001). The high SIRI group tended to stay longer in hospital and lost more blood during surgery. SIRI, body mass index, weight loss, carbohydrate antigen 19-9, radical surgery, and TNM stage were combined to generate a nomogram (C-index, 0.821 in the training cohort, 0.828 in the validation cohort) that was significantly superior to the TNM staging system both in the training (C-index, 0.655) and validation cohort (C-index, 0.649).
Gallbladder cancer (GBC) is a relatively rare tumor type with a poor prognosis [1]. With surgical resection currently the only curative therapy, median survival is approximately 25 months after curative resection according to a multicenter study in the United States conducted in 2016 [2]. It is also a significant source of mortality among countries in Asia and Latin America [3]. As Dr. Fortner and Pack stated in 1958, “the 5-year survival of a patient with GBC constitutes a medical curiosity” [4]. For many decades, physicians have been devoted to making more precise predictions of outcome for patients with GBC. To date, the TNM staging system, which was proposed by the American Joint Committee on Cancer (AJCC), remains the gold standard in cancer management [5]. However, other factors such as patients’ demographic features, symptoms, and laboratory test data may also have important effects on the prognosis of GBC patients, which will lead to great divergence in clinical outcome even in patients at the same TNM stage. Therefore, more accurate and reliable prognostic models for GBC are urgently required in clinical practice.
In cancer development and progression, the inflammatory response is widely recognized as an important factor [6]. Some inflammation-based biomarkers, such as the platelet-to-lymphocyte ratio (PLR), monocyte-to-lymphocyte ratio (MLR), and neutrophil-to-lymphocyte ratio (NLR) have been proposed, and their potential to predict prognosis of GBC has been reported [7]. Recently, a novel inflammation-based biomarker combining peripheral monocytes, neutrophils, and lymphocytes count, named the systemic inflammation response index (SIRI), was proposed by Qi et al. [8], and shows good prognostic ability in some solid tumors including pancreatic, gastric, esophageal, and nasopharyngeal cancer [9-11]. However, there is still no evidence demonstrating whether the SIRI can act as a prognostic indicator to precisely predict GBC patient outcome. Additionally, to the best of our knowledge, there is no prediction model that includes inflammation-based biomarkers for GBC.
Hence, the aims of our study were to investigate the SIRI’s prognostic value using our cohort of patients with GBC, and to construct a prognostic prediction model incorporating the SIRI and test its predictive accuracy.
From 1 December 2003 to 30 June 2017, a total of 124 patients diagnosed as GBC with pathological confirmation after surgical resection at Peking Union Medical College Hospital (PUMCH) in Beijing, China, were retrospectively recruited to this study.
The inclusion criteria were described as follows: (1) GBC confirmed by histopathological examination as the primary diagnosis; (2) surgical resection for GBC performed; (3) routine blood test, serum tumor biomarker test, and infection test results measured within 7 days before surgery; and (4) complete clinicopathological information and postoperative follow-up data available.
The exclusion criteria included: (1) lack of clear histopathological diagnosis; (2) missing clinicopathological information; (3) incomplete follow-up data; (4) other malignant tumors present; and (5) distant metastasis.
Demographic and clinical information were manually reviewed from the medical records. We collected demographic data, symptoms such jaundice, fever, and weight loss, medical history including hypertension and diabetes, serum laboratory test results, physical examination findings, surgical records, and histopathological reports retrospectively. The SIRI was defined as: SIRI=N×M/L, in which N, L, and M refer to peripheral counts of neutrophils, lymphocytes, and monocytes. Since preoperative inflammatory status could affect the results of the complete blood count and thus had effect on the value of the SIRI, we also collected patients’ preoperative inflammation status and antibiotics usage. We defined the combined inflammatory status as one of the following situations: (1) having acute inflammatory disease including acute pancreatitis, acute cholecystitis, or acute cholangitis at admission; (2) body temperature ≥ 37.3°C at admission with white blood cell (WBC) > 10×109/L. GBC stage and postoperative pathological TNM information were determined with the use of the AJCC 8th edition classification system [12]. The maximal tumor size, tumor differentiation grade, and incisional margins were judged based on observations made during surgery and the final histopathological reports. R0 resection was defined as microscopically negative incisional margins. And radical surgery was defined when the radical surgical protocols were carried out and R0 resection was achieved as well. The specific radical surgical protocols were determined by the preoperative staging, operative findings and the results of cryosection biopsy. In details, for patients at the stage Tis or T1a, simple cholecystectomy was performed; for patients at the stage T1b or T2a or T2b, cholecystectomy with > 2 cm hepatic wedge resection were performed; for patients at the stage T3, most of them were received cholecystectomy with en bloc hepatic resection (segments IVB and V), and the other also received extra hemihepatectomy and/or bile duct excision; and for patients at the stage T4, extended radical resection including cholecystectomy, major hepatic resection, peripheral organ resection (omentum, stomach, duodenum, etc.) were performed according to standard radical surgical procedures. For patients at the stage T1b or higher, regional lymphadenectomy was performed. Patients who received palliative surgery were based on one of the following reasons: (1) unresectable disease by operative findings; (2) comorbidity and aging, which could not bear the radical surgery; (3) false cryosection biopsy reports during operation; and (4) declined by the patient’s relatives. The purpose of the palliative surgery was to clarify of diagnosis and relieve of the symptoms such as jaundice and abdominal pain. After discharge, all patients were regularly followed up. The last follow-up time and survival status were recorded. Overall survival (OS) was defined as the interval between the date of surgery and death or the last follow-up time. The last follow-up time was February 2020. After screening, the inclusion criteria were met by 124 patients that were included afterwards.
Categorical variables are presented as numbers and percentages, whereas continuous variables including the SIRI are presented by the median and first and third quartiles. Continuous variables such as body mass index (BMI) and carbohydrate antigen 19-9 (CA19-9) were transformed into categorical variables on the basis of routine cutoff values in the clinical application. The SIRI’s optimal cutoff value for OS was calculated by applying a time-dependent receiver operating characteristic (ROC) analysis. Survival curves were plotted through the Kaplan-Meier method. Log-rank test was used to compare the differences between subgroups. Based on the SIRI cutoff value, patients were divided into high SIRI and low SIRI group. The correlations between different SIRI groups and clinicopathological variables were analyzed by Mann-Whitney U tests or two-sample t tests for continuous variables based on its normality, and by Fisher exact tests or Pearson chi-square tests for categorical variables.
Cox regression methodology was applied for univariate analysis. Variables with a p-value no more than 0.1 in univariate analysis and other potential confounding variables (e.g., the combined inflammatory status and preoperative antibiotics usage) were then subjected to the multivariate Cox proportional hazard regression model. The independent prognostic variables were selected according to the results of Cox proportional analyses. Then, we randomly divided the whole cohort into the nomogram development set and validation set in a proportion of 1:1. A prognostic nomogram was established for predicting OS in the training cohort and Harrell’s concordance index (C-index) was used to measure the predictive accuracy both in the training and the validation cohort. Validation was based on 1,000 bootstrap re-samplings. Nomogram performance was evaluated using calibration plots. The prognostic nomogram was then compared with the traditional TNM staging system using the C-index and decision curve analysis both in the training and the validation cohort [13]. All statistical analyses were performed using SPSS ver. 25.0 (IBM Corp., Armonk, NY) and R 3.6.2 software (Institute of Statistics and Mathematics, Vienna, Austria). A two-sided p-values < 0.05 were considered statistically significant.
The study was approved by the Ethics Committee of PUMCH and CAMS & PUMC (approval decision number: S-K1110) and was conducted according to the ethical standards of the World Medical Association Declaration of Helsinki [14]. In accordance with the committee’s regulations, written informed consent was obtained from all patients who were alive.
A total of 124 patients with GBC were investigated in this study. Study patients’ baseline characteristics are shown in Table 1. Sixty-nine patients (55.6%) were male and 55 (44.4%) were female. The median follow-up time was 20 months (range, 0.5 to 153 months). Twenty (16.1%), 12 (9.7%), nine (7.3%), and 46 (37.1%) patients developed jaundice, fever, fatigue, and weight loss, respectively, when diagnosed with GBC. Elevated preoperative serum CA19-9 was observed in 62 (50%) patients. Before operation for GBC, three patients had acute pancreatitis but no one had acute cholecystitis or cholangitis. Another one patient had elevated body temperature (≥ 37.3°C) with WBC > 10×109/L. In total, four patients were defined to have the combined inflammatory status as previously described. Eight patients had preoperative antibiotics usage. Eighty-five patients (68.5%) were intended to perform radical surgery and 70 of them (82.4%) achieved negative margins (R0 resection). The other 39 patients (31.5%) received palliative surgery. According to the AJCC 8th edition, 39 patients were diagnosed as stage IIIA and 41 patients were stage IIIB. Postoperative histopathological reports revealed that the majority of our patients (119 patients, 96%) had adenocarcinoma, four patients had adenosquamous carcinoma, and one patient had intracholecystic papillary neoplasm. Finally, 79 patients (63.7%) were followed until death.
To calculate the SIRI’s optimal cutoff value, we first generated 1-, 3-, and 5-year time-dependent ROC curves according to the SIRI’s OS, as shown in Fig. 1, with areas under the curve (AUC) of 0.682, 0.636, and 0.666 respectively. Then we determined the SIRI’s optimal cutoff value to be 0.89 using the 1-year time-dependent ROC curve. Based on this cutoff value, we divided the patients into high SIRI and low SIRI groups; the patients’ characteristics of each group are also summarized in Table 1. Compared with the low SIRI group, there were more patients with high CA19-9 (> 40 U/mL) in the high SIRI group (60.3% vs. 37.5%, p=0.019). The surgical margin as R0 tended to be harder to achieve in the high SIRI group (51.5% vs. 82.1%, p=0.001). The patients in the high SIRI group tended to have higher TNM staging distribution (p=0.024). Furthermore, the median follow-up time was significantly shorter in the high SIRI group (12.5 months vs. 27 months, p=0.009), with more patients reaching the state of death (75.0% vs. 50.0%, p=0.005).
To determine whether the SIRI can predict patients’ surgical outcome, we compared the high SIRI group with the low SIRI group in terms of length of stay in hospital, hemorrhage during surgery, the change in Barthel score after surgery, and different kinds of complications including bleeding, infection, liver failure, biliary fistula, ascites, and others (Table 2). The patients in the high SIRI group tended to stay longer in hospital as compared with the low SIRI group (16 days vs. 12 days, p=0.007). Furthermore, during surgery, patients in the high SIRI group lost more blood than those in the low SIRI group, though the median blood losses were equal (200 mL vs. 200 mL, p=0.001). However, no significant differences were observed in the change in Barthel score and the incidence rates of all types of postoperative complications. Combining the occurrence of all the complications, there was still no significant difference between the high and low SIRI groups (p=0.286).
The Kaplan-Meier curves of OS according to the SIRI showed significant difference, as confirmed by the log-rank test (p < 0.001) (Fig. 2). Moreover, upon univariate analysis, BMI ≥ 24, jaundice, weight loss, CA19-9 > 40 U/mL, radical surgery, TNM stage, tumor differentiation grade, and SIRI showed statistically significant associations with OS. Multivariate analysis revealed that BMI < 24 (hazard ratio [HR], 2.008; 95% confidence interval [CI], 1.162 to 3.471; p=0.013), weight loss (HR, 1.729; 95% CI, 1.004 to 2.976; p=0.048), high SIRI (HR, 1.753; 95% CI, 1.027 to 2.991; p=0.040), CA19-9 > 40 U/mL (HR, 2.162; 95% CI, 1.194 to 3.916; p=0.011), no radical surgery (HR, 2.940; 95% CI, 1.676 to 5.159; p < 0.001), and TNM stage (III-IV) (HR, 7.523; 95% CI, 1.558 to 36.329; p=0.012) were independent factors for OS (Table 3).
We first randomly equally divided the patients into the training cohort and the validation cohort. In order to build the prognostic model for OS prediction in the training cohort, the resulting variables from the multivariate Cox analysis were included. The prognostic factors included six risk factors, including BMI, weight loss, SIRI, CA19-9, radical surgery, and TNM stage. The time-dependent ROC curves of each included factor and the prognostic model are shown in Fig. 3, which showed that the SIRI’s AUCs were higher than BMI and weight loss, but lower than CA19-9, radical surgery, TNM stage. Furthermore, the prognostic model combining all six factors had better predictive accuracy than any of the single factors, with AUCs of 1, 3, and 5 years as 0.897, 0.912, and 0.913, respectively. To visualize the prognostic model and make it more practical, a nomogram containing these six factors was constructed (Fig. 4). Each factor in the nomogram was assigned with a point based on its status. Summing the total points from all variables and drawing a vertical line at the location of the total points scale allowed us to predict the probabilities of the outcomes in terms of 1-, 3-, and 5-year OS probability.
The model’s predictive ability was assessed by calculating the C-index, which was 0.821 (95% CI, 0.759 to 0.883) in the training cohort and 0.828 (95% CI, 0.762 to 0.894), demonstrating the nomogram’s good predictive accuracy. Additionally, the nomogram’s performance was graphically evaluated by making 1- and 3-year calibration plots (Fig. 5). The predicted line was very close to the reference line both in the training and the validation cohort, which indicates the model’s good performance. Finally, to test the clinical usefulness of our model, decision curve analysis was performed (Fig. 6). Compared with the TNM staging system, our model offered much better clinical utility.
We developed histograms of the nomogram-predicted probability of 12-month survival according to different AJCC TNM staging groupings. As shown in Fig. 7, in patients with TNM stage IIIA and IIIB, there was a wide variety in the distribution of nomogram-predicted probabilities, ranging from 0.05 to 0.95. Additionally, the C-index of the AJCC TNM system was 0.655 (95% CI, 0.592 to 0.718) in the training cohort and 0.649 (95% CI, 0.582 to 0.716) in the validation cohort, which was significantly lower than our prognostic model both in the training and the validation cohort.
GBC is the most common malignancy in the biliary tract system [15,16]. Although surgical resection is a potentially curative therapy, over one-third of patients experience a recurrence [17]. Moreover, approximately 40% of cases are diagnosed at advanced stages [18,19]. All these factors contribute to its current poor prognosis situation, with only 10%-25% patients achieving 5-year survival [20]. Thus, the accuracy of prognosis prediction for different patients is crucial for physicians to make better clinical decisions. To date, the AJCC TNM staging system for both diagnosis and prognosis in GBC remains the gold standard; however, its own limitations in terms of its poor discrimination of the heterogeneity among patients at the same TNM stage are still unsolvable. To address this problem, we recognized the SIRI, a novel inflammation-based index, as an independent significant prognostic indicator and developed a novel prediction model combining the SIRI and other clinicopathological factors that has a much better predictive ability compared with the TNM staging system.
As Hanahan and Weinberg [21] proposed, cancer-related inflammation is one of the hallmarks of cancer, and plays a vital role in carcinogenesis and tumor progression. Based on this theory, some indices using peripheral immune and inflammatory cells, such as PLR, MLR, and NLR, have been developed and their utility in survival prediction has been proven [22-24]. In 2016, Qi et al. [8] described a new inflammation-based index, the SIRI, which showed good prognostic value in patients with pancreatic cancer. In our study, we found that a high SIRI correlated to poor prognosis in respect of OS. Previous research has shown similar results in other solid tumors including gastric, esophageal, and nasopharyngeal cancer [9-11]. However, the SIRI’s optimal cutoff differed among studies, at 1.8 in pancreatic cancer and at 1.2 in esophageal cancer [8,10]. We adopted 0.89 as the optimal cutoff to stratify our groups of patients, but whether it is applicable to all GBC patients requires further validation. Additionally, our results showed that the SIRI can also predict patients’ surgical outcome such as length of stay in hospital and hemorrhage during surgery. To be more specific, patients in the high SIRI group may indicate the procedure’s higher difficulty, which sheds new light on the SIRI regarding its potential in the assessment of surgical difficulty and therefore enabling individual peri-operative care decisions.
The specific mechanisms as to why a high SIRI indicates a poor outcome in GBC patients remain unclear; however, previous studies have revealed that lymphocytes have a vital function in anti-tumor defense in their role as infiltrating tumor cells, inducing cancer cell apoptosis [25]. Furthermore, the peripheral monocyte count has association with the level of tumor-associated macrophages, which facilitate tumor cell development and suppress the immune system against them [26,27]. Similarly, several studies suggested that peripheral neutrophils have a role in providing a favorable microenvironment for tumor growth, invasion, and metastasis by means of secreting different types of cytokines including intercellular adhesion molecule 1 [28]. Taken together, either increasing neutrophils and monocytes or decreasing lymphocytes will cause an elevation in the SIRI, which will lead to a rather worse prognosis for patients with cancer. Finally, since the SIRI is easily calculated from the results of complete blood count tests, it is applicable to test the SIRI frequently during follow-up. Both the SIRI’s value and dynamic changes may have the potential to serve as a marker to evaluate the efficacy of adjuvant chemoradiotherapy, to select appropriate patients to receive specific targeted therapy and immune therapy, and also to monitor for possible recurrence, although further investigations are needed.
In the present study, we took advantage of the user-friendly graphical interfaces of the nomogram to demonstrate our prediction model based on the SIRI and other clinicopathological factors including BMI, weight loss, CA19-9, radical surgery, and TNM stage. Among these factors, CA19-9 and TNM stage have also been utilized in other prognostic models of GBC [29,30]. BMI and weight loss are also easily acquired information, demonstrating our model’s convenience. Though jaundice is significant in univariate analysis, it is not significant in multivariate analysis. Further studies should investigate the prognostic role of jaundice in larger cohorts. Compared with the traditional TNM staging system, our prediction model had better discriminatory ability, consistency, and clinical utility, as shown in the C-index, calibration plot, and decision curve analysis. Therefore, this prognostic model is appropriate for predicting GBC patient prognosis after surgery, which will be useful in helping clinicians with clinical counseling, decision-making, and follow-up planning. To the best of our knowledge, this is the first report to create a nomogram combining inflammatory indices including the SIRI and other indicators to predict OS probability in GBC patients.
Our study has several limitations. First, it was based on a retrospective cohort from a single center, which may cause potential selection bias. Second, the number of patients included in this study is relatively small; thus, further external validation is required before our results can be applied in other institutions. Third, this study only focused on the SIRI and other clinicopathological factors, and other inflammation-related indexes such as C-reactive protein, liver function test results, and coagulation were not investigated.
In conclusion, this is the first study to show that the SIRI is an independent predictor of OS in GBC patients. Our prediction model combining the SIRI and other clinicopathological indicators performed well in predicting patient’s survival probability, surpassing the traditional TNM staging system regarding its predictive accuracy. It has the potential to serve as a practical clinical tool for individualized prognostication.
ACKNOWLEDGMENTS
This work was supported by grants from CAMS Innovation Fund for Medical Sciences (CIFMS) (No.2016-I2M-1-001) and Tsinghua University-Peking Union Medical College Hospital Cooperation Project (PTQH201904552).
References
1. de Aretxabala X. Biliary spillage a new prognostic factor in gallbladder cancer? Hepatobiliary Surg Nutr. 2019; 8:537–8.

2. Buettner S, Margonis GA, Kim Y, Gani F, Ethun CG, Poultsides GA, et al. Changing odds of survival over time among patients undergoing surgical resection of gallbladder carcinoma. Ann Surg Oncol. 2016; 23:4401–9.

3. Wi Y, Woo H, Won YJ, Jang JY, Shin A. Trends in gallbladder cancer incidence and survival in Korea. Cancer Res Treat. 2018; 50:1444–51.

4. Fortner JG, Pack GT. Clinical aspects of primary carcinoma of the gallbladder. AMA Arch Surg. 1958; 77:742–50.

5. Sung YN, Song M, Lee JH, Song KB, Hwang DW, Ahn CS, et al. Validation of the 8th edition of the American Joint Committee on Cancer staging system for gallbladder cancer and implications for the follow-up of patients without node dissection. Cancer Res Treat. 2020; 52:455–68.

6. Diakos CI, Charles KA, McMillan DC, Clarke SJ. Cancer-related inflammation and treatment effectiveness. Lancet Oncol. 2014; 15:e493–503.

7. Cho KM, Park H, Oh DY, Kim TY, Lee KH, Han SW, et al. Neutrophil-to-lymphocyte ratio, platelet-to-lymphocyte ratio, and their dynamic changes during chemotherapy is useful to predict a more accurate prognosis of advanced biliary tract cancer. Oncotarget. 2017; 8:2329–41.

8. Qi Q, Zhuang L, Shen Y, Geng Y, Yu S, Chen H, et al. A novel systemic inflammation response index (SIRI) for predicting the survival of patients with pancreatic cancer after chemotherapy. Cancer. 2016; 122:2158–67.

9. Li S, Lan X, Gao H, Li Z, Chen L, Wang W, et al. Systemic Inflammation Response Index (SIRI), cancer stem cells and survival of localised gastric adenocarcinoma after curative resection. J Cancer Res Clin Oncol. 2017; 143:2455–68.

10. Geng Y, Zhu D, Wu C, Wu J, Wang Q, Li R, et al. A novel systemic inflammation response index (SIRI) for predicting postoperative survival of patients with esophageal squamous cell carcinoma. Int Immunopharmacol. 2018; 65:503–10.

11. Chen Y, Jiang W, Xi D, Chen J, Xu G, Yin W, et al. Development and validation of nomogram based on SIRI for predicting the clinical outcome in patients with nasopharyngeal carcinomas. J Investig Med. 2019; 67:691–8.

12. Amin MB, Greene FL, Edge SB, Compton CC, Gershenwald JE, Brookland RK, et al. The eighth edition AJCC cancer staging manual: continuing to build a bridge from a population-based to a more “personalized” approach to cancer staging. CA Cancer J Clin. 2017; 67:93–9.

13. Vickers AJ, Elkin EB. Decision curve analysis: a novel method for evaluating prediction models. Med Decis Making. 2006; 26:565–74.

14. General Assembly of the World Medical Association. World Medical Association Declaration of Helsinki: ethical principles for medical research involving human subjects. J Am Coll Dent. 2014; 81:14–8.
15. Narayan RR, Creasy JM, Goldman DA, Gonen M, Kandoth C, Kundra R, et al. Regional differences in gallbladder cancer pathogenesis: insights from a multi-institutional comparison of tumor mutations. Cancer. 2019; 125:575–85.

17. Margonis GA, Gani F, Buettner S, Amini N, Sasaki K, Andreatos N, et al. Rates and patterns of recurrence after curative intent resection for gallbladder cancer: a multi-institution analysis from the US Extra-hepatic Biliary Malignancy Consortium. HPB (Oxford). 2016; 18:872–8.

18. Li M, Liu F, Zhang F, Zhou W, Jiang X, Yang Y, et al. Genomic ERBB2/ERBB3 mutations promote PD-L1-mediated immune escape in gallbladder cancer: a whole-exome sequencing analysis. Gut. 2019; 68:1024–33.

19. Yang P, Javle M, Pang F, Zhao W, Abdel-Wahab R, Chen X, et al. Somatic genetic aberrations in gallbladder cancer: comparison between Chinese and US patients. Hepatobiliary Surg Nutr. 2019; 8:604–14.

20. Primrose JN, Fox RP, Palmer DH, Malik HZ, Prasad R, Mirza D, et al. Capecitabine compared with observation in resected biliary tract cancer (BILCAP): a randomised, controlled, multicentre, phase 3 study. Lancet Oncol. 2019; 20:663–73.

22. Chen YM, Lai CH, Chang HC, Chao TY, Tseng CC, Fang WF, et al. Baseline and trend of lymphocyte-to-monocyte ratio as prognostic factors in epidermal growth factor receptor mutant non-small cell lung cancer patients treated with first-line epidermal growth factor receptor tyrosine kinase inhibitors. PLoS One. 2015; 10:e0136252.

23. Cheng H, Luo G, Lu Y, Jin K, Guo M, Xu J, et al. The combination of systemic inflammation-based marker NLR and circulating regulatory T cells predicts the prognosis of resectable pancreatic cancer patients. Pancreatology. 2016; 16:1080–4.

24. Diem S, Schmid S, Krapf M, Flatz L, Born D, Jochum W, et al. Neutrophil-to-lymphocyte ratio (NLR) and platelet-to-Lymphocyte ratio (PLR) as prognostic markers in patients with non-small cell lung cancer (NSCLC) treated with nivolumab. Lung Cancer. 2017; 111:176–81.

26. Shibutani M, Maeda K, Nagahara H, Fukuoka T, Nakao S, Matsutani S, et al. The peripheral monocyte count is associated with the density of tumor-associated macrophages in the tumor microenvironment of colorectal cancer: a retrospective study. BMC Cancer. 2017; 17:404.

27. Franklin RA, Liao W, Sarkar A, Kim MV, Bivona MR, Liu K, et al. The cellular and molecular origin of tumor-associated macrophages. Science. 2014; 344:921–5.

28. Liu S, Li N, Yu X, Xiao X, Cheng K, Hu J, et al. Expression of intercellular adhesion molecule 1 by hepatocellular carcinoma stem cells and circulating tumor cells. Gastroenterology. 2013; 144:1031–41.

Fig. 1.
Time-dependent receiver operating characteristic (ROC) analysis of systemic inflammation response index (SIRI) for 1-, 3-, and 5-year survival. AUC, area under the curve.
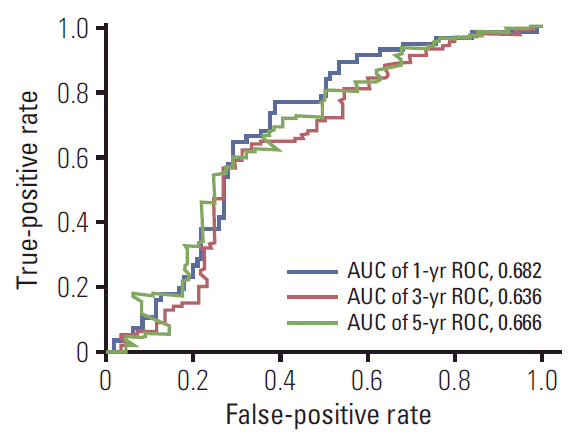
Fig. 2.
Kaplan-Meier survival curves of different systemic inflammation response index (SIRI) groups.
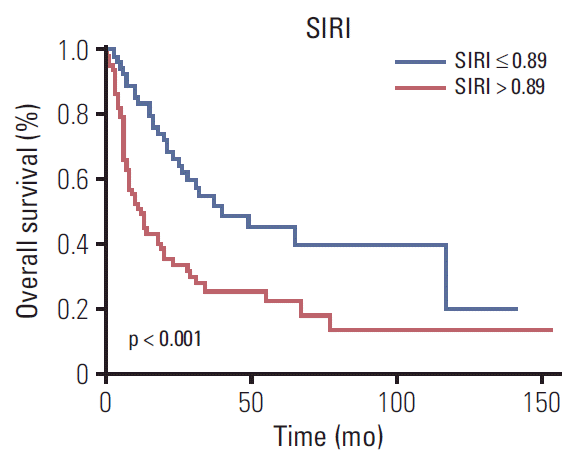
Fig. 3.
Time-dependent receiver operating characteristic analysis of each of the selected factors and the prognostic model. SIRI, systemic inflammation response index; BMI, body mass index; CA19-9, carbohydrate antigen 19-9; AUC, area under the curve.
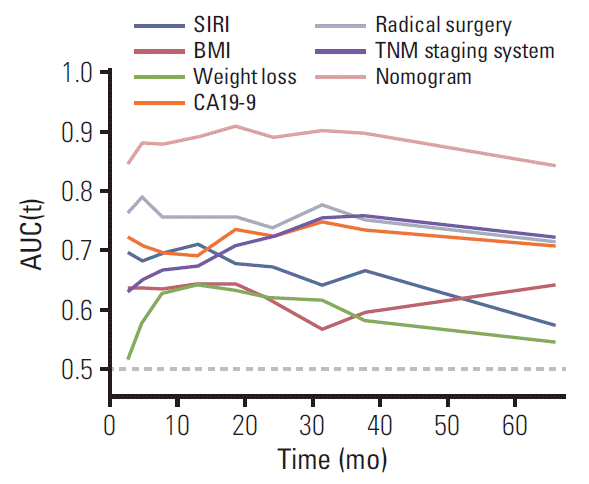
Fig. 4.
Prognostic nomogram for predicting 1-, 3-, and 5-year overall survival probability based on the systemic inflammation response index (SIRI) group, body mass index (BMI), weight loss, carbohydrate antigen 19-9 (CA19-9), radical surgery, and TNM stage in patients with gallbladder cancer.
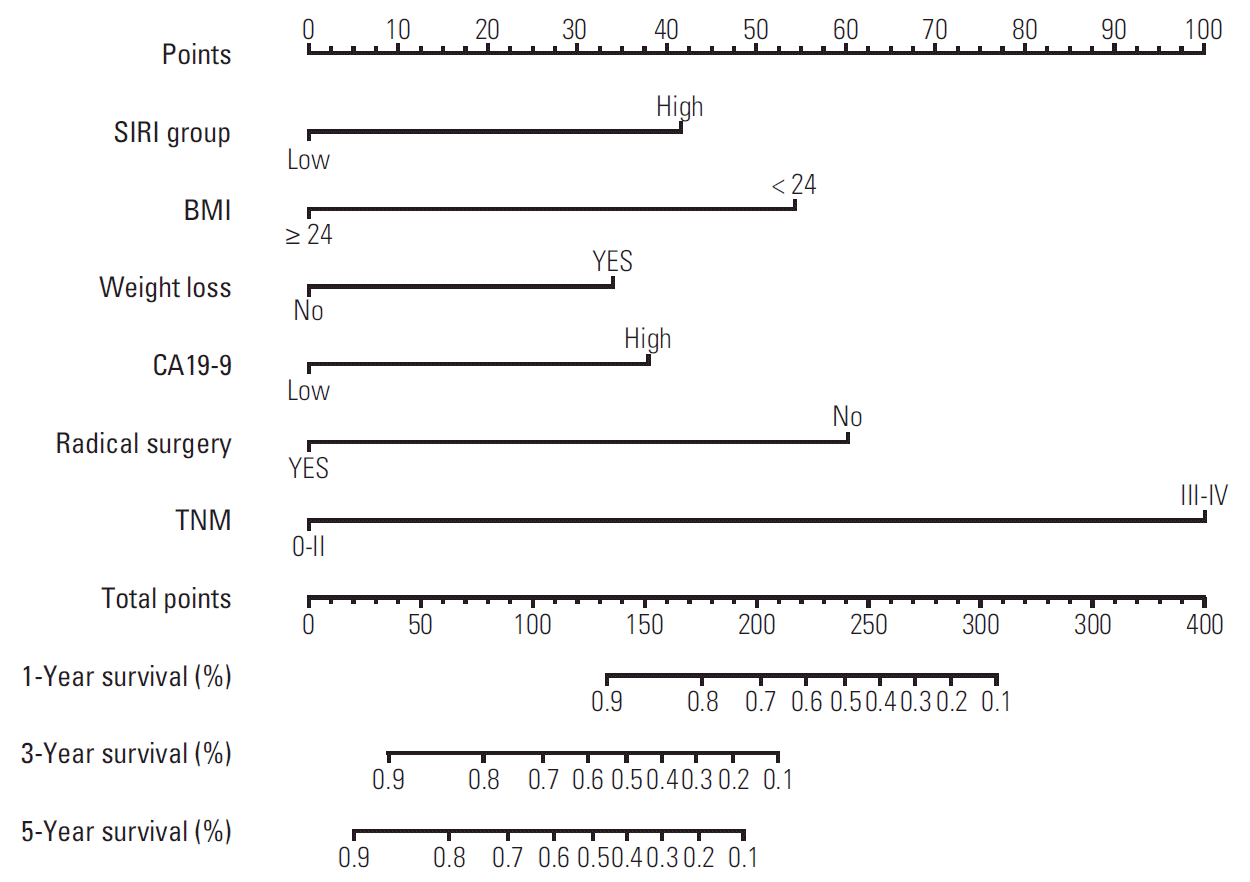
Fig. 5.
Nomogram calibration plot for predicting overall survival probabilities at 1 year (A, C) and 3 years (B, D).
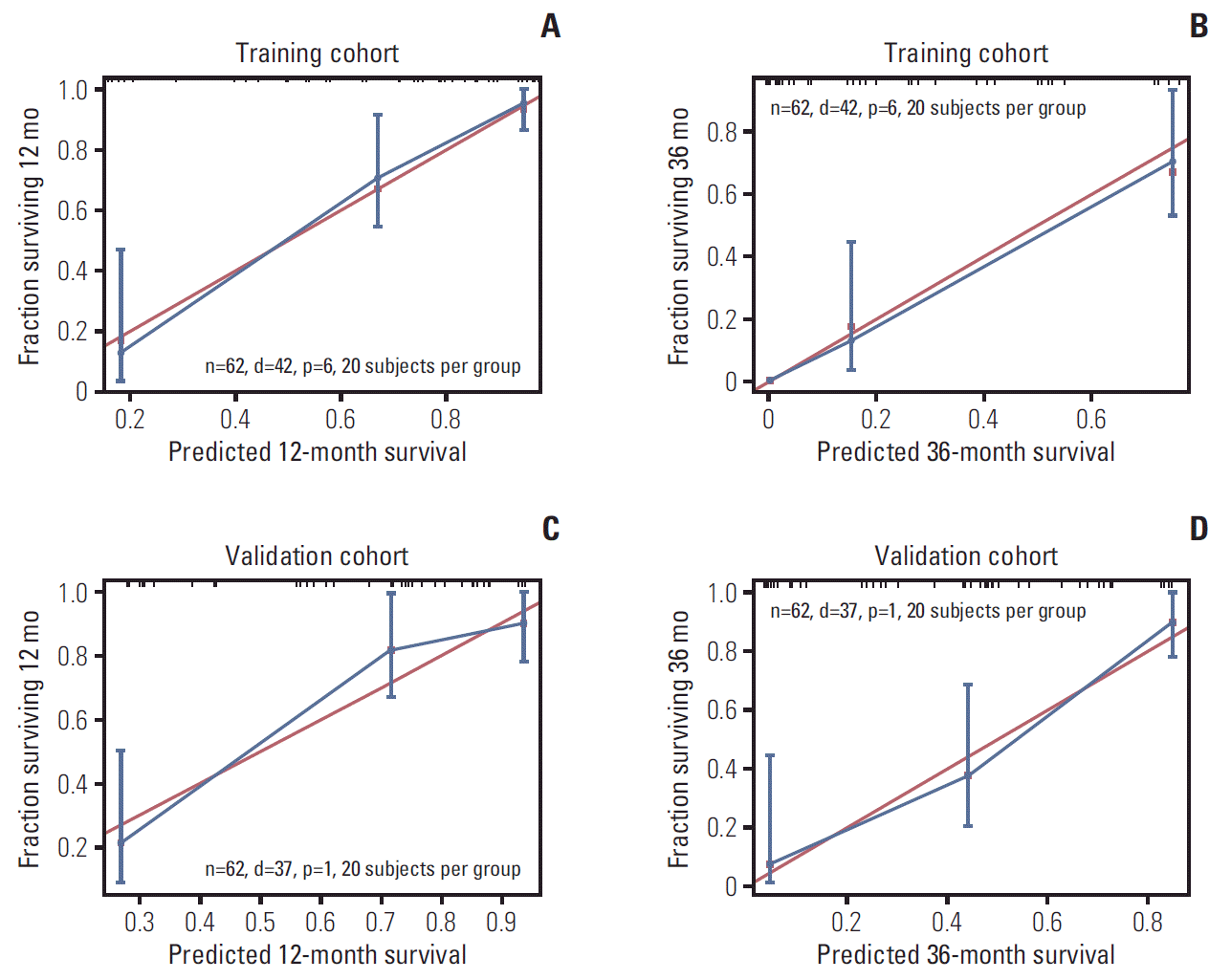
Fig. 6.
Decision curve analysis (DCA) of the model and TNM staging system for 1- (A, B), 3- (C, D), and 5-year (E, F) overall survival (OS).
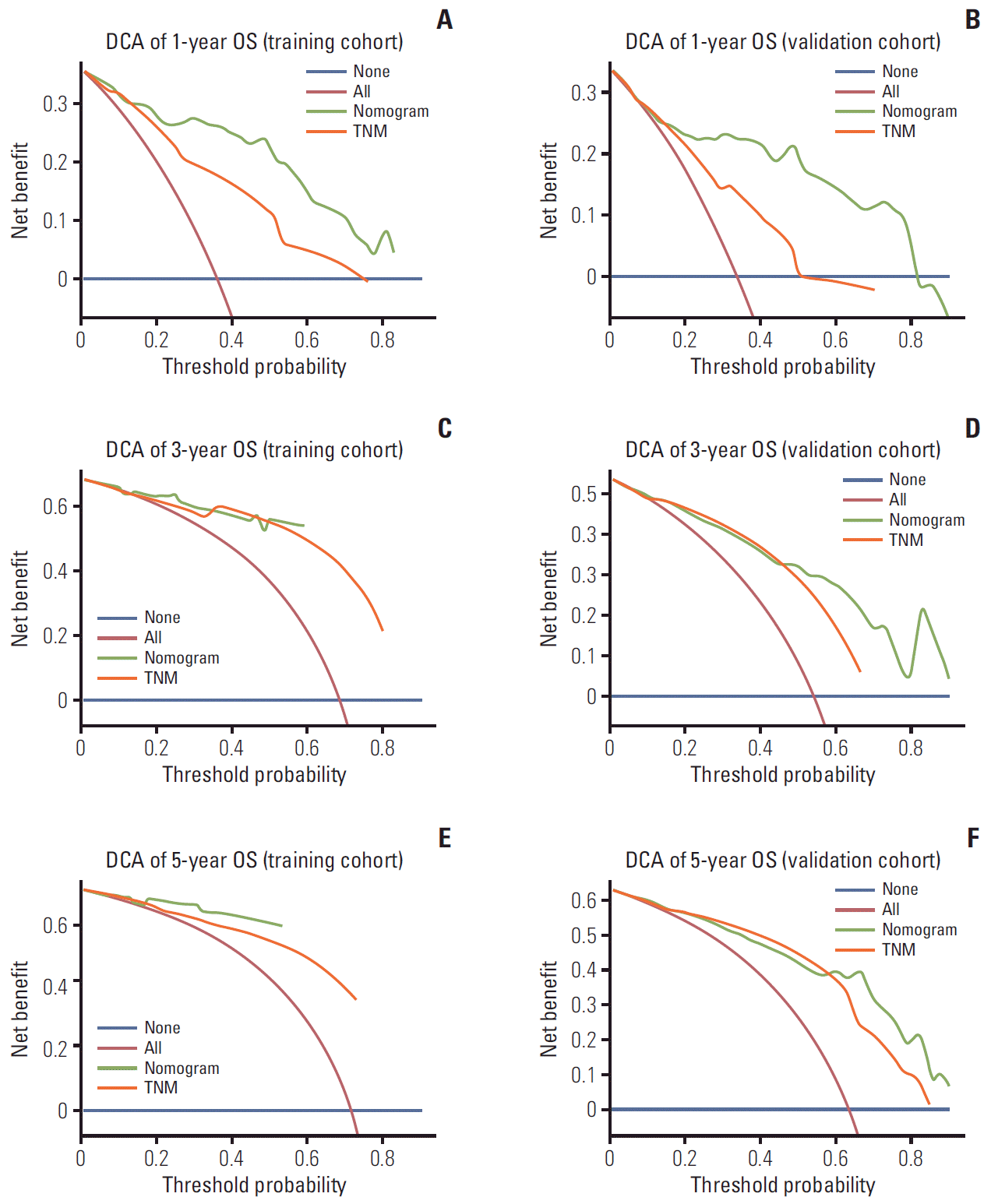
Fig. 7.
Histograms of nomogram-predicted probability of 12-month survival according to the different American Joint Committee on Cancer TNM stage groupings.
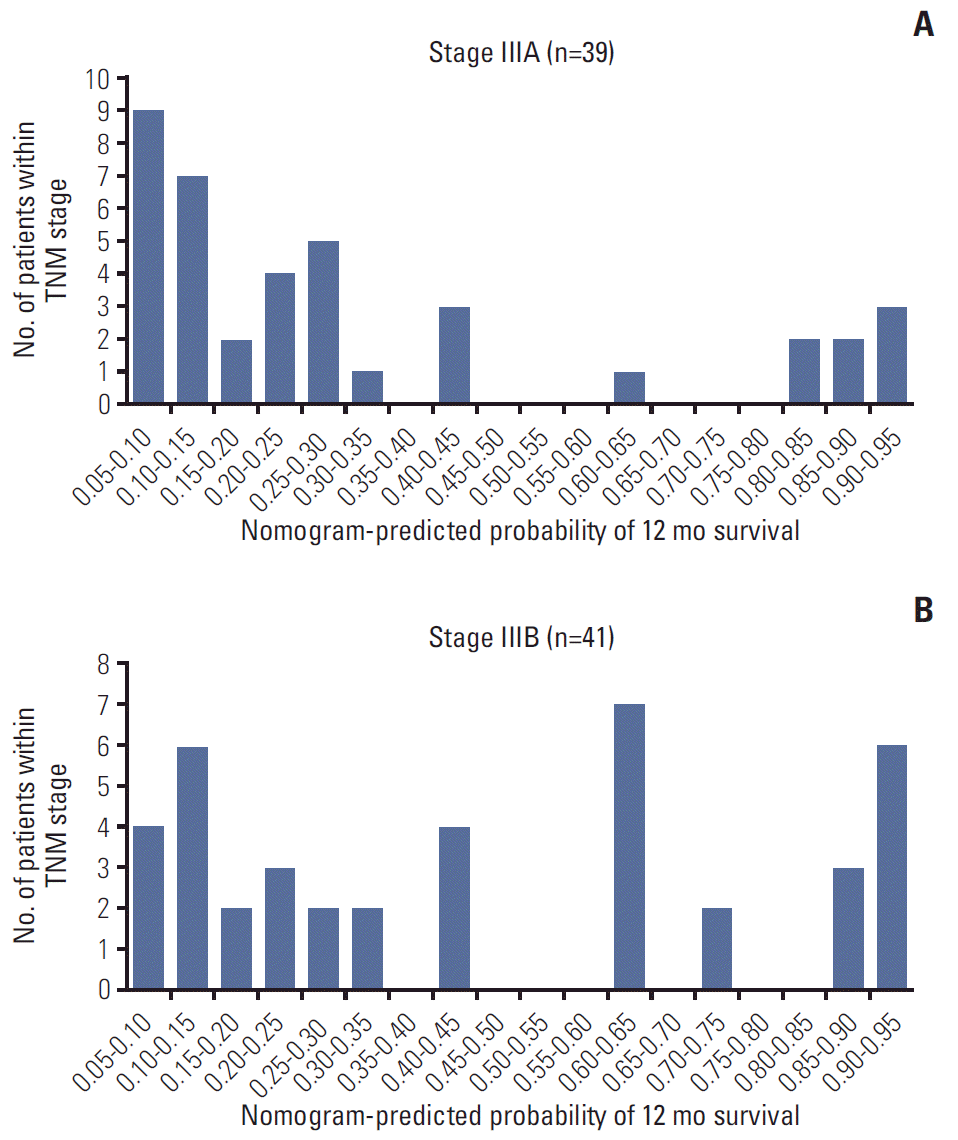
Table 1.
Baseline characteristics of all patients
Table 2.
The relationship between the SIRI and surgical outcome
Table 3.
Univariate and multivariate Cox proportional hazards analysis for OS in 124 patients with GBC




 PDF
PDF Citation
Citation Print
Print


 XML Download
XML Download