1. Sankaranarayanan R, Masuyer E, Swaminathan R, Ferlay J, Whelan S. Head and neck cancer: a global perspective on epidemiology and prognosis. Anticancer Res. 1998; 18:4779–86.
2. Epstein JB, Gorsky M, Guglietta A, Le N, Sonis ST. The correlation between epidermal growth factor levels in saliva and the severity of oral mucositis during oropharyngeal radiation therapy. Cancer. 2000; 89:2258–65.

3. Leborgne JH, Leborgne F, Zubizarreta E, Ortega B, Mezzera J. Corticosteroids and radiation mucositis in head and neck cancer: a double-blind placebo-controlled randomized trial. Radiother Oncol. 1998; 47:145–8.

4. Rosenthal DI, Trotti A. Strategies for managing radiation-induced mucositis in head and neck cancer. Semin Radiat Oncol. 2009; 19:29–34.

5. Hille A, Rave-Frank M, Pradier O, Damm C, Dorr W, Jackel MC, et al. Effect of keratinocyte growth factor on the proliferation, clonogenic capacity and colony size of human epithelial tumour cells in vitro. Int J Radiat Biol. 2003; 79:119–28.

6. Klaunig JE, Wang Z, Pu X, Zhou S. Oxidative stress and oxidative damage in chemical carcinogenesis. Toxicol Appl Pharmacol. 2011; 254:86–99.

7. Zafarullah M, Li WQ, Sylvester J, Ahmad M. Molecular mechanisms of N-acetylcysteine actions. Cell Mol Life Sci. 2003; 60:6–20.
8. Sheng-Tanner X, Bump EA, Hedley DW. An oxidative stress-mediated death pathway in irradiated human leukemia cells mapped using multilaser flow cytometry. Radiat Res. 1998; 150:636–47.

9. Ishii T, Itoh K, Yamamoto M. Roles of Nrf2 in activation of antioxidant enzyme genes via antioxidant responsive elements. Methods Enzymol. 2002; 348:182–90.
10. Chan K, Lu R, Chang JC, Kan YW. NRF2, a member of the NFE2 family of transcription factors, is not essential for murine erythropoiesis, growth, and development. Proc Natl Acad Sci USA. 1996; 93:13943–8.

11. Zhang J. Teaching the basics of autophagy and mitophagy to redox biologists: mechanisms and experimental approaches. Redox Biol. 2015; 4:242–59.
12. Shin YS, Shin HA, Kang SU, Kim JH, Oh YT, Park KH, et al. Effect of epicatechin against radiation-induced oral mucositis: in vitro and in vivo study. PLoS One. 2013; 8:e69151.

13. Zhu W, Xu J, Ge Y, Cao H, Ge X, Luo J, et al. Epigallocatechin-3-gallate (EGCG) protects skin cells from ionizing radiation via heme oxygenase-1 (HO-1) overexpression. J Radiat Res. 2014; 55:1056–65.

14. Koike M, Shiomi T, Koike A. Identification of skin injury-related genes induced by ionizing radiation in human keratinocytes using cDNA microarray. J Radiat Res. 2005; 46:173–84.

15. Won HR, Kang SU, Kim HJ, Jang JY, Shin YS, Kim CH. Non-thermal plasma treated solution with potential as a novel therapeutic agent for nasal mucosa regeneration. Sci Rep. 2018; 8:13754.

16. Cha HY, Lee BS, Chang JW, Park JK, Han JH, Kim YS, et al. Downregulation of Nrf2 by the combination of TRAIL and valproic acid induces apoptotic cell death of TRAIL-resistant papillary thyroid cancer cells via suppression of Bcl-xL. Cancer Lett. 2016; 372:65–74.

17. Li XY, Zhao Y, Sun MG, Shi JF, Ju RJ, Zhang CX, et al. Multifunctional liposomes loaded with paclitaxel and artemether for treatment of invasive brain glioma. Biomaterials. 2014; 35:5591–604.

18. Lomax ME, Folkes LK, O’Neill P. Biological consequences of radiation-induced DNA damage: relevance to radiotherapy. Clin Oncol (R Coll Radiol). 2013; 25:578–85.

19. Yamamori T, Yasui H, Yamazumi M, Wada Y, Nakamura Y, Nakamura H, et al. Ionizing radiation induces mitochondrial reactive oxygen species production accompanied by upregulation of mitochondrial electron transport chain function and mitochondrial content under control of the cell cycle checkpoint. Free Radic Biol Med. 2012; 53:260–70.

20. McDonald JT, Kim K, Norris AJ, Vlashi E, Phillips TM, Lagadec C, et al. Ionizing radiation activates the Nrf2 antioxidant response. Cancer Res. 2010; 70:8886–95.

21. Yang Y, Yang Y, Yang X, Zhu H, Guo Q, Chen X, et al. Autophagy and its function in radiosensitivity. Tumour Biol. 2015; 36:4079–87.

22. Erwin-Toth P. Skin changes from radiation therapy. J Wound Ostomy Continence Nurs. 2007; 34:546.

23. Sonis ST. The biologic role for nuclear factor-kappaB in disease and its potential involvement in mucosal injury associated with anti-neoplastic therapy. Crit Rev Oral Biol Med. 2002; 13:380–9.

24. Renzing J, Hansen S, Lane DP. Oxidative stress is involved in the UV activation of p53. J Cell Sci. 1996; 109(Pt 5):1105–12.

25. Lambros MP, Kondapalli L, Parsa C, Mulamalla HC, Orlando R, Pon D, et al. Molecular signatures in the prevention of radiation damage by the synergistic effect of N-acetyl cysteine and qingre liyan decoction, a traditional chinese medicine, using a 3-dimensional cell culture model of oral mucositis. Evid Based Complement Alternat Med. 2015; 2015:425760.

26. Kim CH, Kang SU, Pyun J, Lee MH, Hwang HS, Lee H. Epicatechin protects auditory cells against cisplatin-induced death. Apoptosis. 2008; 13:1184–94.

27. Shin YS, Cha HY, Lee BS, Kang SU, Hwang HS, Kwon HC, et al. Anti-cancer effect of luminacin, a marine microbial rxtract, in head and neck dquamous cell carcinoma progression via autophagic cell death. Cancer Res Treat. 2016; 48:738–52.
28. Tsukimoto M, Tamaishi N, Homma T, Kojima S. Low-dose gamma-ray irradiation induces translocation of Nrf2 into nuclear in mouse macrophage RAW264.7 cells. J Radiat Res. 2010; 51:349–53.

29. Muriach M, Flores-Bellver M, Romero FJ, Barcia JM. Diabetes and the brain: oxidative stress, inflammation, and autophagy. Oxid Med Cell Longev. 2014; 2014:102158.

30. Fang C, Gu L, Smerin D, Mao S, Xiong X. The Interrelation between reactive oxygen apecies and autophagy in neurological disorders. Oxid Med Cell Longev. 2017; 2017:8495160.
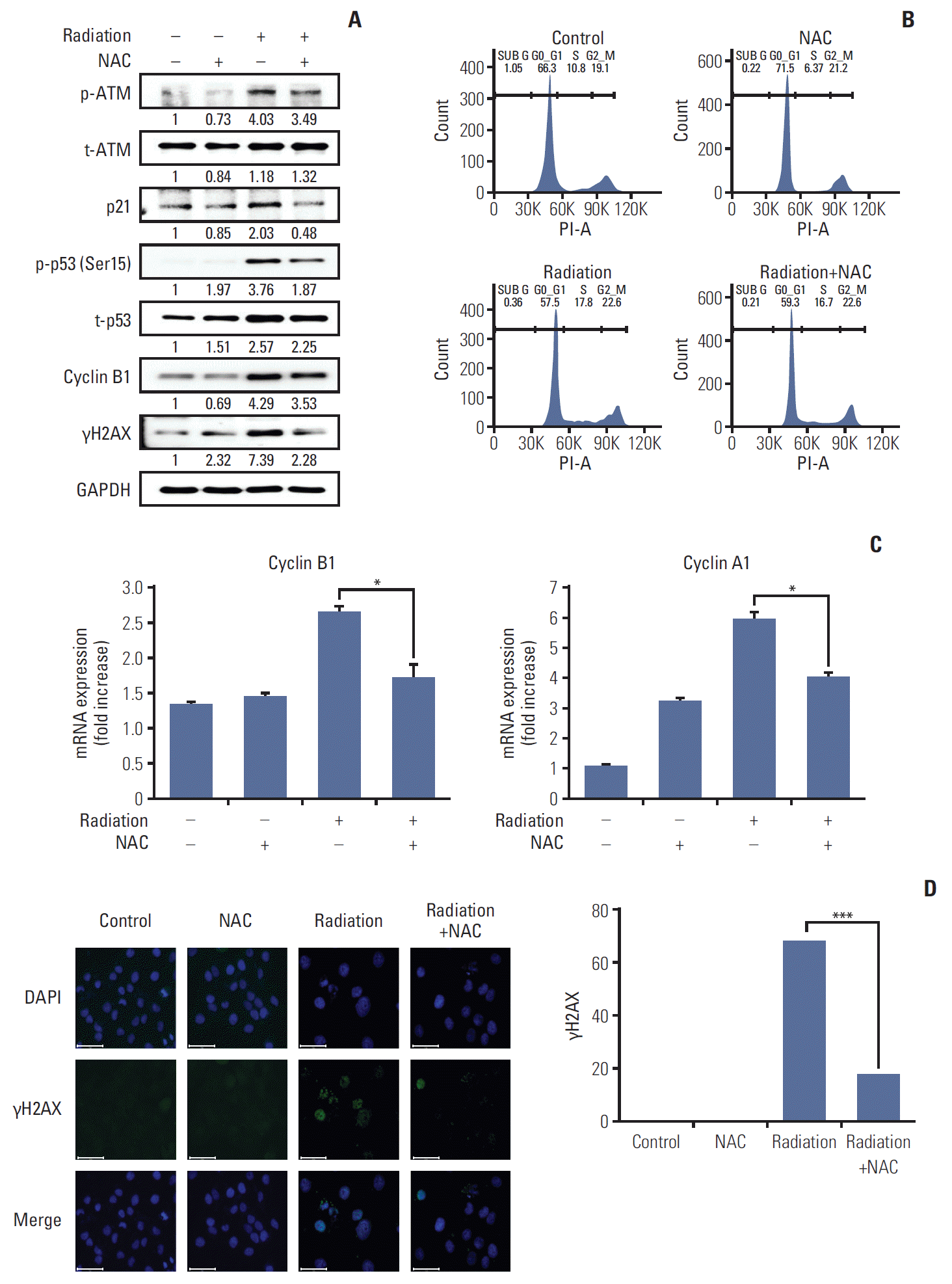
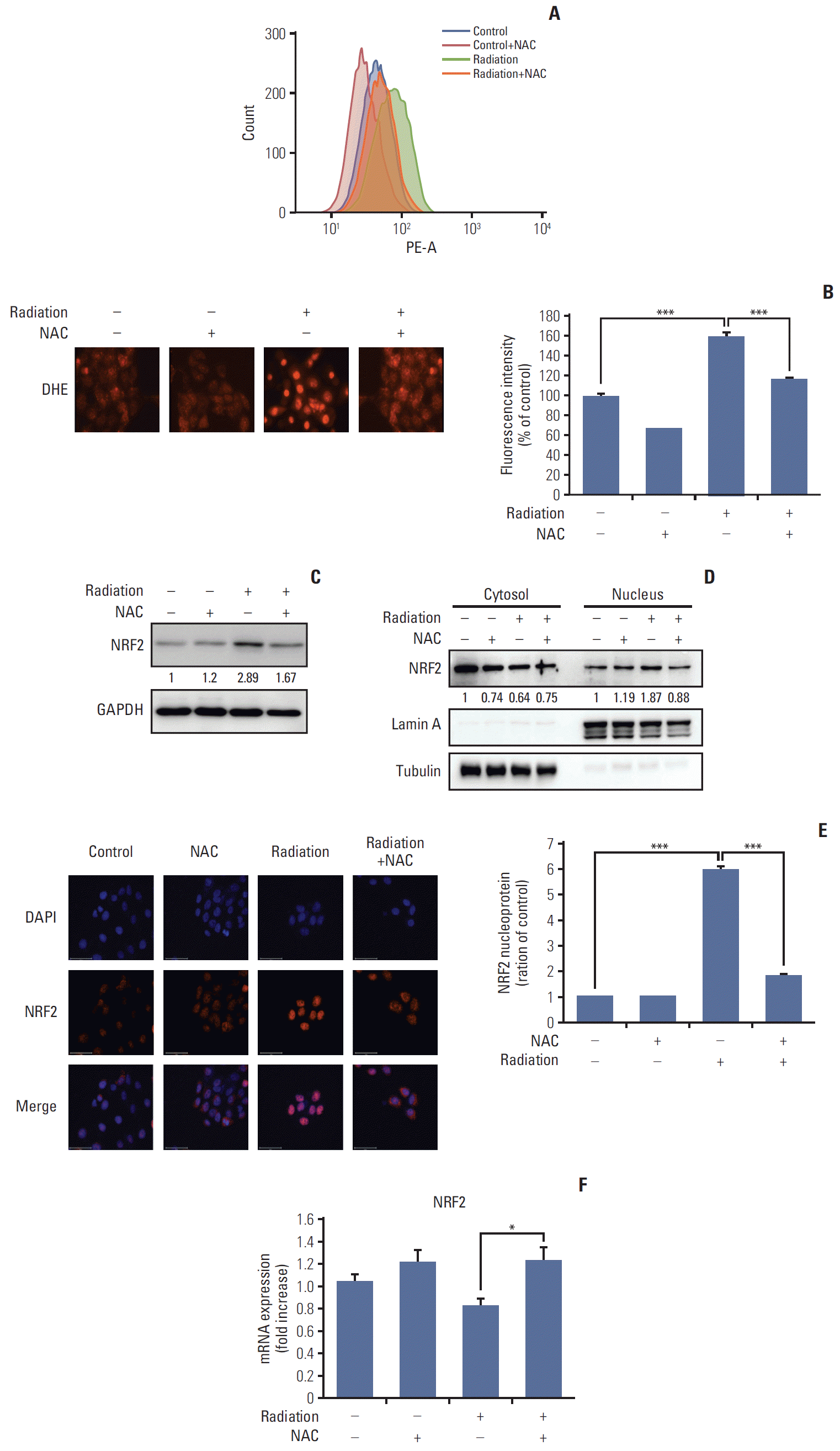
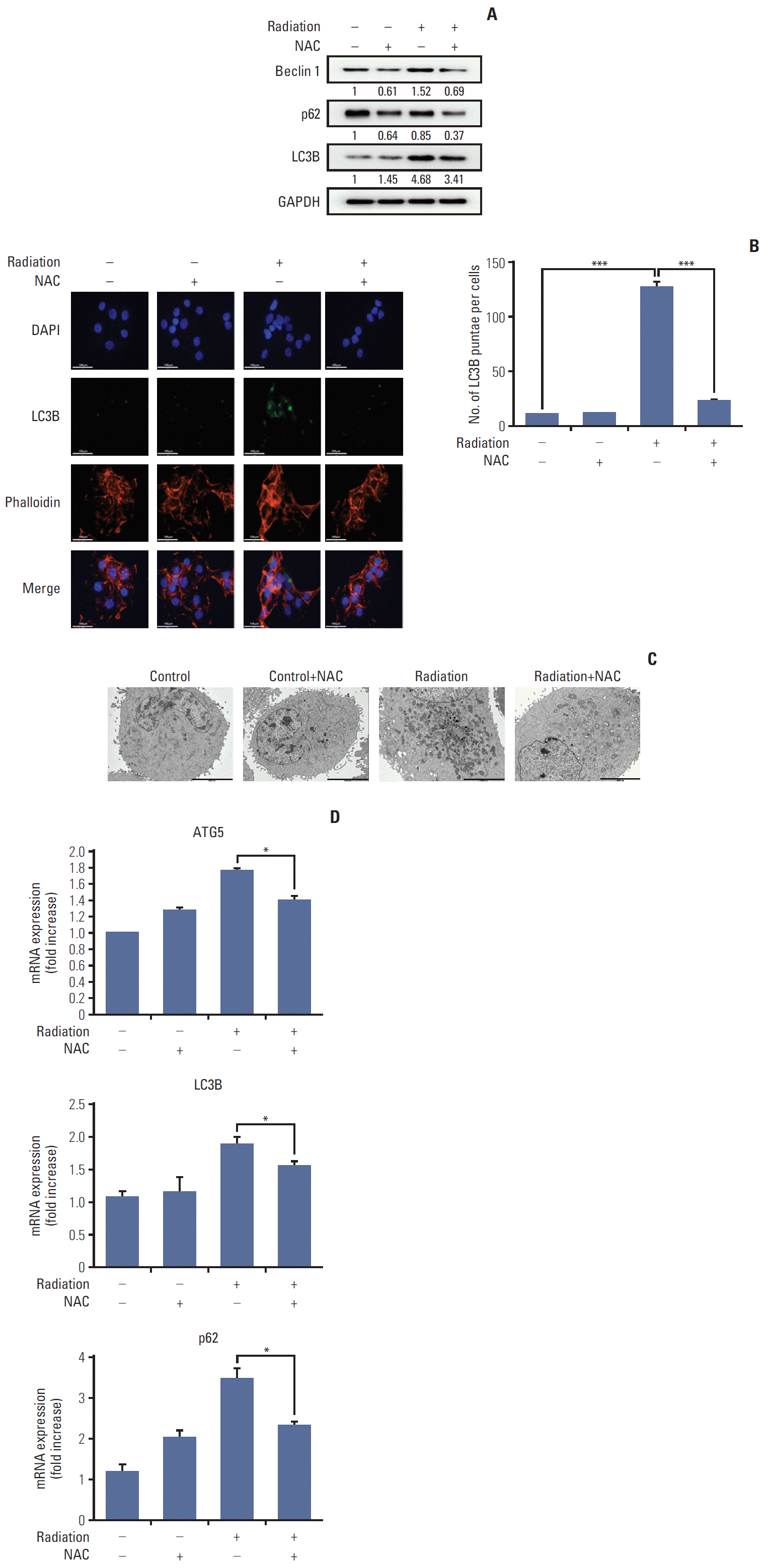
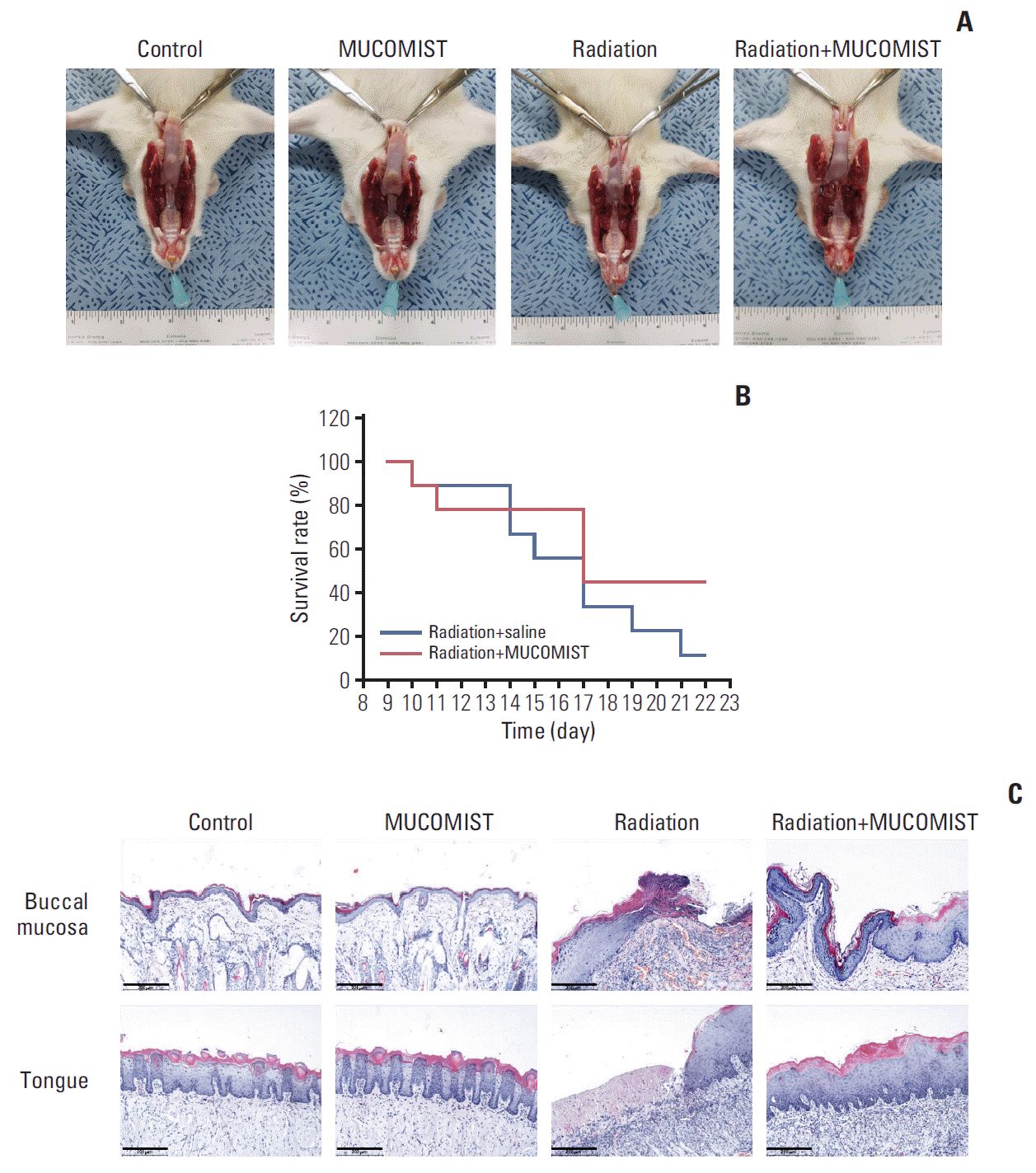




 PDF
PDF Citation
Citation Print
Print


 XML Download
XML Download