Introduction
Materials and Methods
1. Mice and cell lines
2. Peptides, antibodies, and reagents
3. Production of recombinant retroviruses encoding costimulatory ligands
4. Transduction of recombinant retroviruses encoding costimulatory ligands into in vitro–activated T cells
5. In vitro proliferation assays
6. Immunization
7. Evaluation of immune responses
8. Evaluation of antitumor effects
9. Single-cell preparation
10. Statistical analyses
11. Ethical statement
Results
1. Induction of antigen-specific CD8 T cell responses with genetically modified CD8 T cell
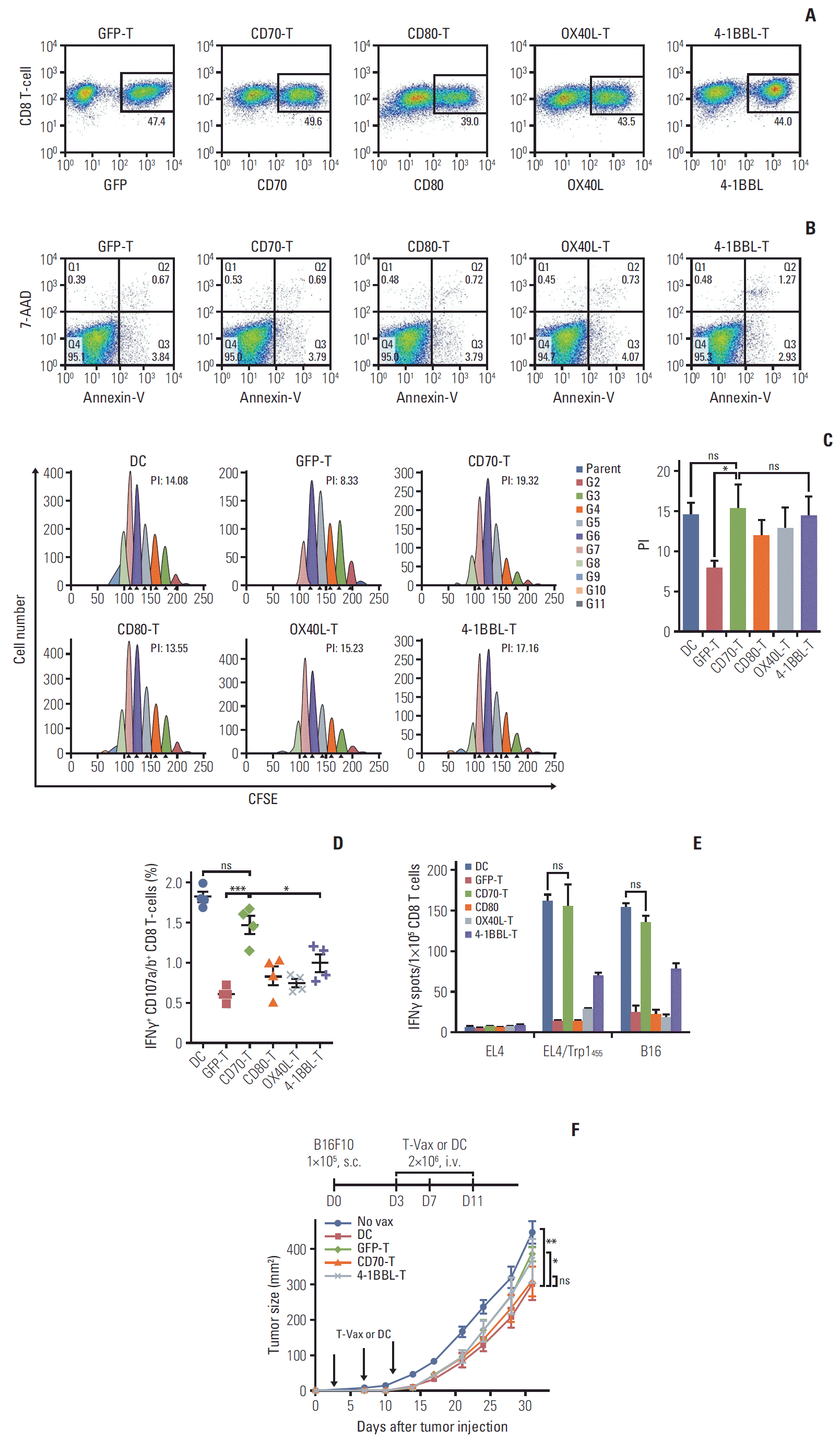 | Fig. 1.Genetically modified CD8 T-cell–based vaccination induces antigen-specific CD8 T-cell responses comparable to dendritic cell-based vaccination. Ex vivo activated splenocytes were transduced with recombinant retroviruses encoding mouse CD70, CD80, OX40L, and 4-1BBL (CD70-T, CD80-T, OX40L-T, and 4-1BBL-T, respectively). GFP was used as a mockcontrol (GFP-T). (A) The expression of transduced CD8 T cells was examined on day 3 post-transduction. (B) Cell apoptosis of the transduced were assessed by staining with annexin-V and 7-aminoactinomycin D (7-AAD) on day 3 post-transduction. (C) Proliferation of Pmel-1 T cells in response to genetically modified CD8 T cells. Pmel-1 T cells were labeled with 5 μM carboxyfluorescein succinimidyl ester and cultured with conditioned CD8 T cells as indicated, including DCs which were loaded with 1 μg/mL hgp10025 peptide. On day 3 post-co-culture, cell proliferation was measured by flow cytometry and analyzed using Modifit LT software (left panel). The results are the sum of the average proliferation index (PI) of Pmel-1 T cells from three independent experiments with standard deviation (SD, bars) of the means (right panel). PI: the sum of the cells in all generations divided by the computed number of parental cells present at the start of experiment. CFSE, carboxyfluorescein succinimidylester; ns, not significant; *p < 0.05. (D, F) B6 mice (2 per group) were immunized intravenously (i.v.) on day 0, 4, and 8 with 2×106 Trp1455/9M-loaded conditioned CD8 T cells as indicated. Antigen-loaded dendritic cells (DC) and GFP-T were used for comparison. (D) Seven days after the last immunization, frequency of antigen-specific CD8 T cells in spleen from individual mouse was evaluated by cell surface mobilization of CD107a/b and intracellular interferon γ (IFNγ) staining. The results are the sum of two independent experiments. Points and bars indicate values for each individual mouse and SD, respectively. (E) Freshly isolated CD8 T cells from pooled splenocytes in D were evaluated for antigen-induced IFNγ secretions by EliSpot against un-pulsed (EL4) and peptide-pulsed EL4 (EL4/Trp1455), and B16 tumor cells. Results represent the average number of spots from duplicate wells with SD (bars) of the means. p-values were calculated using unpaired Student’s t test (ns, not significant; *p < 0.05, ***p < 0.001). (F) Therapeutic efficacy of Trp1455/9M-loaded conditioned CD8 T cells against 3-day-established B16 melanoma. B6 mice (5 per group) were inoculated subcutaneously (s.c.) with 1×105 B16 cells on day 0 and received 2×106 Trp1455/9M-loaded DCs or CD8 T cells on day 3, 7, and 11 (vertical arrows). Non-vaccinated mice (No vax) were included as controls. Tumor size was determined in individual mice by measurements of two opposing diameters and are presented as tumor areas in mm2. Points and bars indicate mean for each group of mice and SD, respectively. p-values were calculated using 2-way ANOVA test (ns, not significant; *p < 0.05, **p < 0.01). These experiments were repeated twice with similar results. |
2. Effects of peptide-based booster Immunization after priming with peptide-loaded CD70-T Vaccines
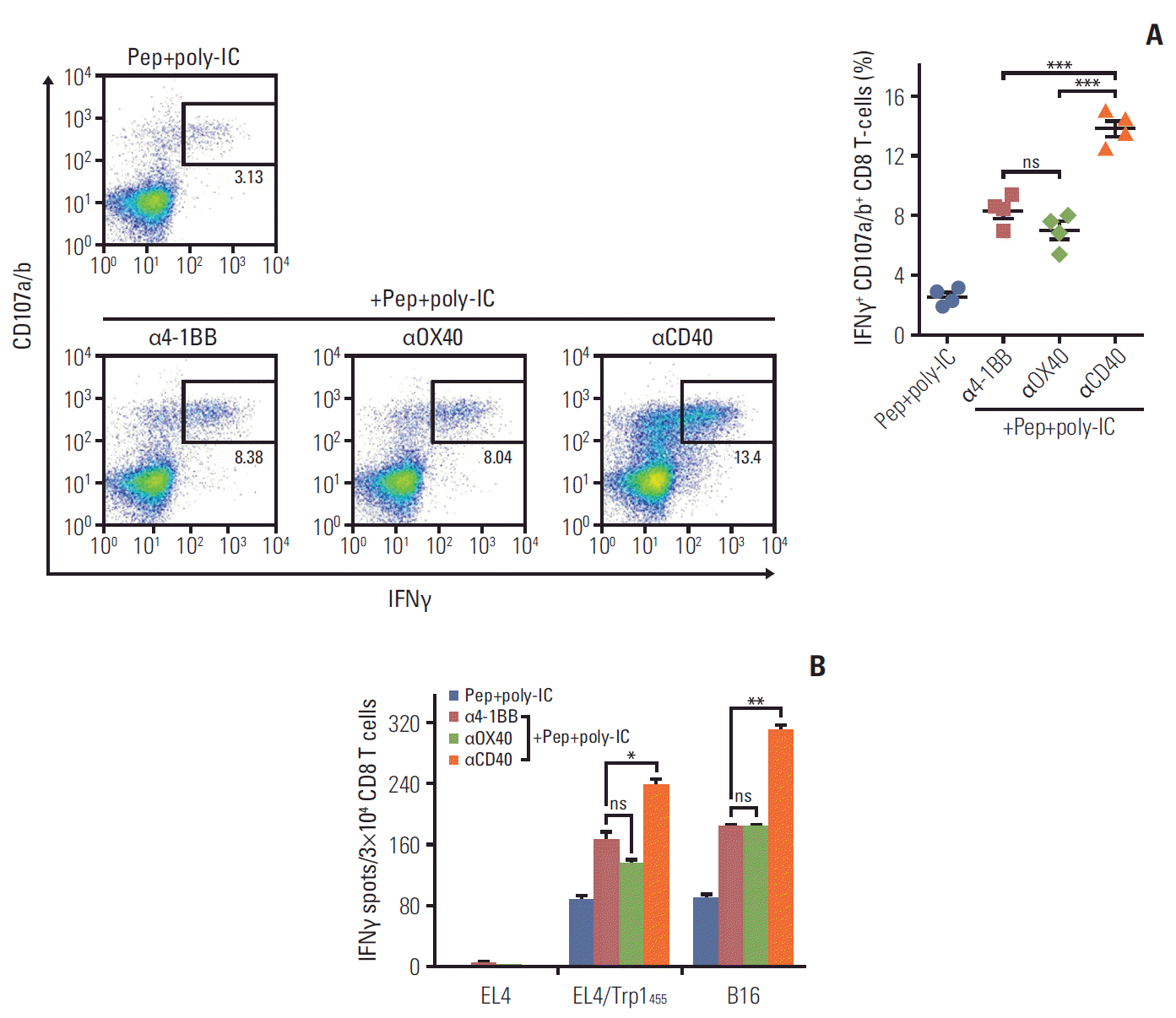 | Fig. 2.Peptide-based booster immunization after priming with CD70-T cells drives enhanced CD8 T-cell responses. B6 mice (2 per group) were immunized intravenously on day 0 with 2×106 Trp1455/9M-loaded CD70-T cells; 7 days later, the mice received a booster immunization with various combination of 100 μg of Trp1455/9M peptide, 50 μg of poly-IC, and 50 μg anti-CD40, anti–4-1BB, or anti-OX40 antibodies. (A) Seven days after last immunization, the frequency of antigen-specific CD8 T cells in spleen was evaluated as in Fig. 1D. A representative dot plot analysis for one mouse of each group is presented (left panel). Numbers in each rectangular gate represent the percentage of interferon γ (IFNγ) and cell surface CD107a/b double-positive cells of all CD8 T cells. The results are the sum of two independent experiments. Points and bars indicate values for each individual mouse and standard deviation (SD), respectively. (B) Antigen-induced IFNγ-secretions with freshly isolated CD8 T cells from pooled splenocytes in A were evaluated as in Fig. 1E. Results represent the average number of spots from duplicate wells with SD (bars) of the means. p-values were calculated using unpaired Student’s t test (ns, not significant; *p < 0.05, **p < 0.01, ***p < 0.001). These experiments were repeated twice with similar results. |
3. Therapeutic antitumor effects of CD70-T prime_TriVax boost vaccination against established B16 melanoma
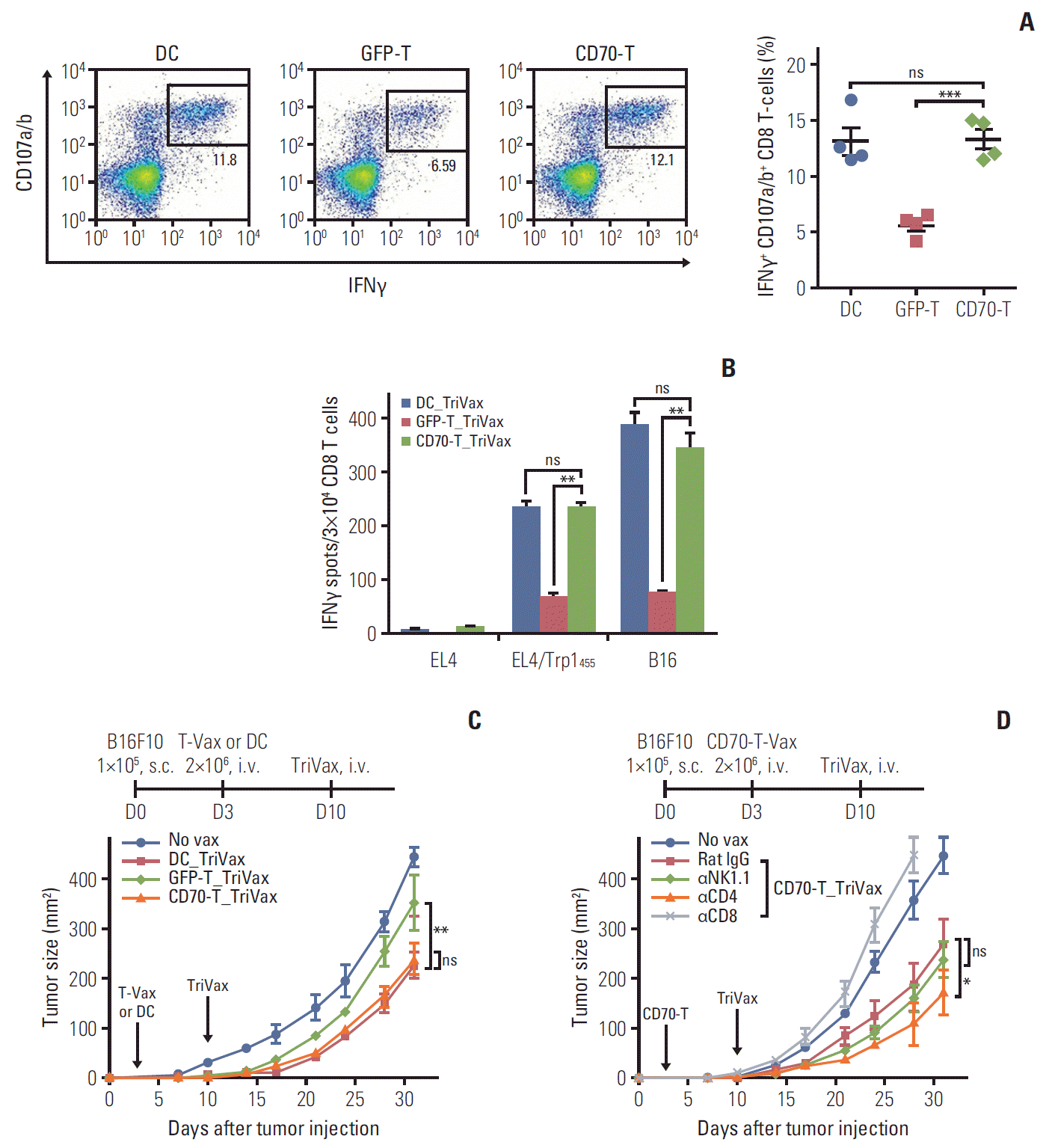 | Fig. 3.Therapeutic antitumor effects of CD70-T prime_TriVax boost vaccination against established B16 melanoma. B6 mice (2 per group) were immunized intravenously (i.v.) on day 0 with 2×106 Trp1455/9M-loaded GFP-T or CD70-T cells; 7 days later, the mice received a booster immunization with combination of 100 μg of Trp1455/9M peptide, 50 μg of poly-IC, and 50 μg anti-CD40 antibodies (GFP-T_TriVax and CD70-T_TriVax, respectively). Trp1455/9M-loaded dendritic cells prime_TriVax boost (DC_TriVax) were included for comparison. (A) Seven days after last immunization, the frequency of antigen-specific CD8 T cells in spleen was evaluated as in Fig. 1D. A representative dot plot analysis for one mouse of each group is presented (left panel). Numbers in each rectangular gate represent the percentage of interferon γ (IFNγ) and cell surface CD107a/b double-positive cells of all CD8 T cells. The results are the sum of two independent experiments. Points and bars indicate values for each individual mouse and standard deviation (SD), respectively. (B) Antigen-induced IFNγ-secretions with freshly isolated CD8 T cells from pooled splenocytes in A were evaluated as in Fig. 1E. Results represent the average number of spots from duplicate wells with SD (bars) of the means. p-values were calculated using unpaired Student’s t test (ns, not significant; **p < 0.01, ***p < 0.001). (C) Therapeutic efficacy of CD70-T_TriVax vaccination against 3-day-established B16 melanoma. B6 mice (5 per group) were inoculated subcutaneously (s.c.) with 1×105 B16 cells on day 0 and received 2×106 Trp1455/9M-loaded GFP-T or CD70-T cells on day 3. After 7 days, the mice received a TriVax booster immunization (vertical arrows). Non-vaccinated mice (No vax) and DC_TriVax were included for comparison. (D) Effector mechanism of CD70-T_TriVax vaccination involved in antitumor effects. B6 mice (5 per group) were administered intraperitoneally with indicated antibodies –1 and –3 days before each immunization. No vax and Rat IgG-treated mice were included as controls. Tumor sizes were determined in individual mice by measurements of two opposing diameters and are presented as tumor areas in mm2. Points and bars indicate the mean for each group of mice and SD, respectively. p-values were calculated using 2-way ANOVA test (ns, not significant; *p < 0.05, **p < 0.01). These experiments were repeated twice with similar results. |
4. Enhanced therapeutic efficacy of CD70-T_TriVax vaccination along with PD-L1 blockade
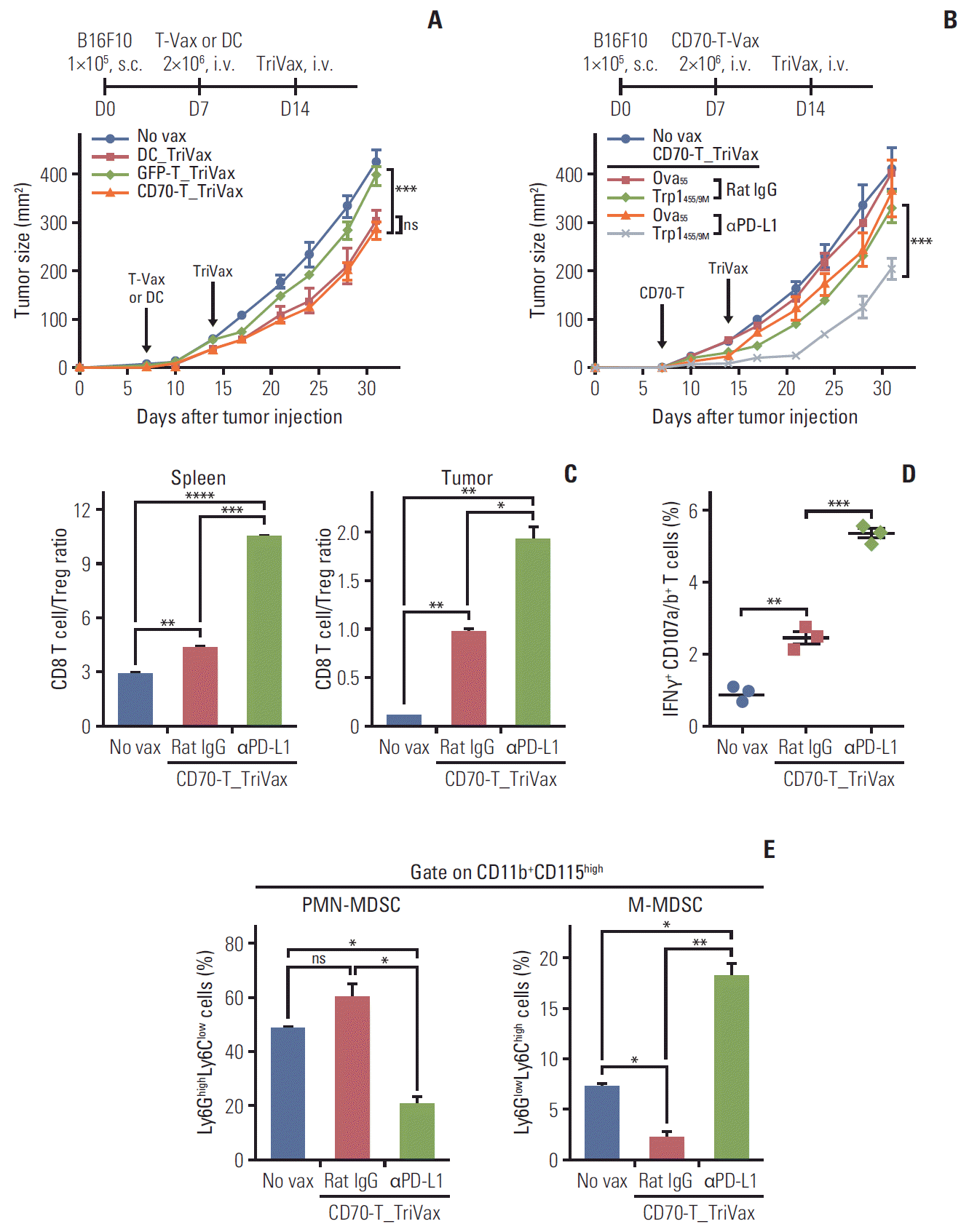 | Fig. 4.Programmed death-ligand 1 (PD-L1) blockade augments the therapeutic efficacy of CD70-T_TriVax vaccination. (A) Therapeutic efficacy of CD70-T_TriVax vaccination against advanced B16 melanoma. B6 mice (7 per group) were inoculated subcutaneously on day 0 with 1×105 B16 cells, followed by vaccination with 2×106 GFP-T or CD70-T cells on day 7, and TriVax on day 14 (vertical arrows). Non-vaccinated mice (No vax) and DC_TriVax were included for comparison. (B) PD-L1 blockade enhanced the efficacy of CD70-T_TriVax vaccination regimen. B6 mice (5 per group) were inoculated subcutaneously (s.c.) on day 0 with 1×105 B16 cells and were immunized as described in A. Rat IgG (as controls) and anti–PD-L1 antibodies were administered intraperitoneally on days +1 and +3 after each immunization. No vaccinated (No vax) and Ova55-loaded CD70-T_TriVax vaccinated mice (Ova55) were included for comparison. Tumor sizes were determined in individual mice by measurements of two opposing diameters and are presented as tumor areas in mm2. Points and bars indicate the mean for each group of mice and standard deviation (SD), respectively. p-values were calculated using 2-way ANOVA test (ns, not significant; ***p < 0.001). (C-E) In a parallel with B, mice (3 per group) were sacrificed on day 21, and the CD8 T-cell responses and immune cell populations were evaluated. Cells from disaggregated tissues of spleen and tumor were analyzed for the composition of various subsets of immune cells. (C) CD8 T cell/Treg ratio as measured by percentage of CD8+ T cells per percentage of CD4+ Foxp3+ T cells cells in each group. (D) The frequency of antigen-specific CD8 T cells in spleen was evaluated as in Fig. 1D. Points and SD indicate values for each individual mouse and SD, respectively. ns, not significant; *p < 0.05, **p < 0.01, ***p < 0.001, ****p < 0.0001. (E) Quantification of Ly6GlowLy6Chigh monocytic myeloid-derived suppressor cells (M-MDSC) and Ly6GhighLy6Clow granulocytic polymorphonuclear MDSC (PMN-MDSC) gated on CD11b+CD115highmyeloid cells in tumor sites. Results represent the average percentage of the gated cells from individual mice with SD (bars) of the means. p-values were calculated using unpaired Student’s t test (ns, not significant; *p < 0.05, **p < 0.01. These experiments were repeated twice with similar results. |
5. Effective antitumor CD8 T cell response of multi-epitope-loaded CD70-T_TriVax immunization
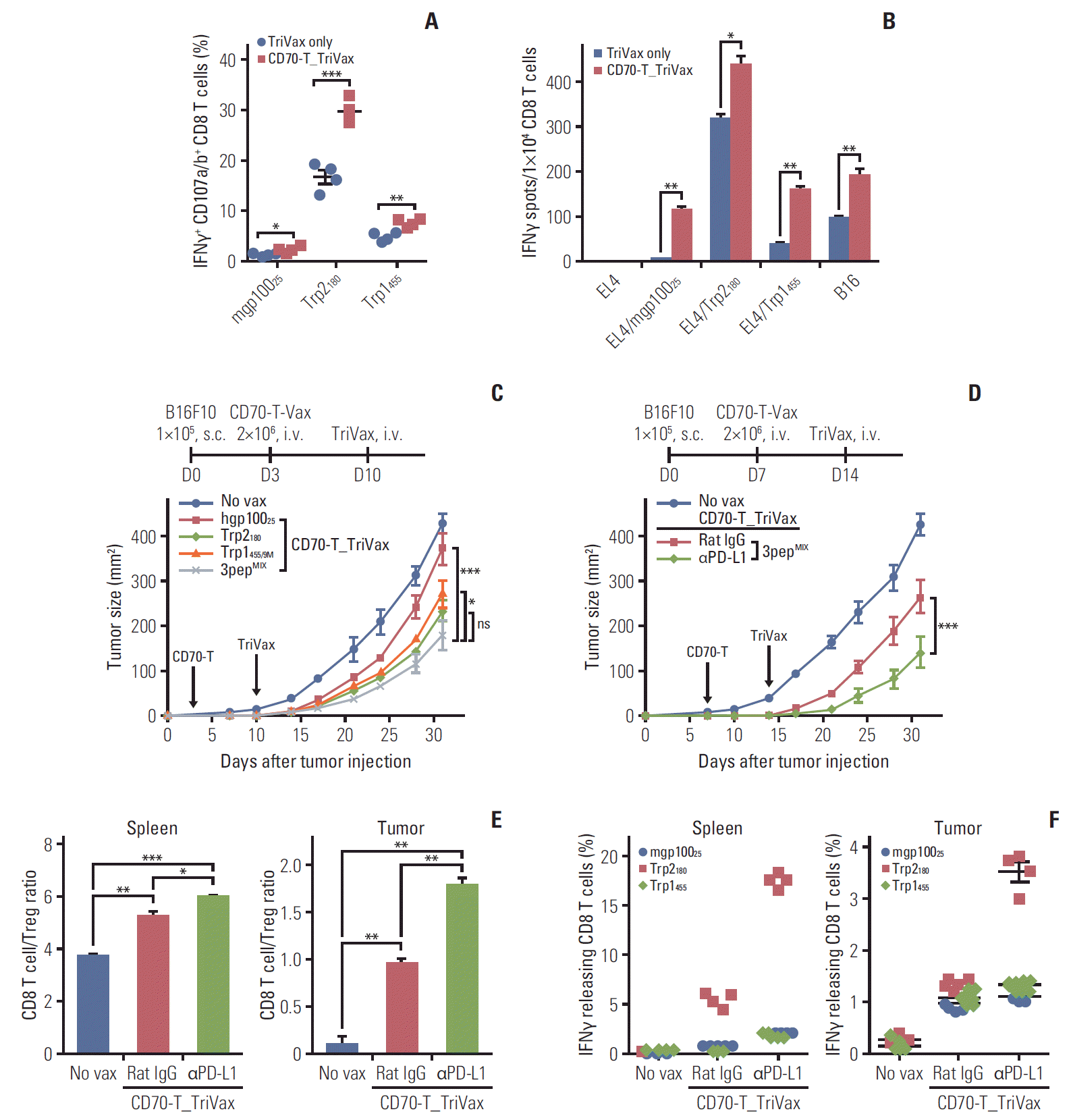 | Fig. 5.Multi-epitope-loaded CD70-T cells priming followed by TriVax booster immunization induces concurrent effective antitumor CD8 T-cell responses. B6 mice (2 per group) were immunized intravenously (i.v.) with CD70-T cells that were loaded with mixture of Trp1455/9M, Trp2180, and hgp10025 peptides; 7 days later, the mice received 3pepMIXTriVax. (A) The frequency of antigen-specific CD8 T cells in spleen was evaluated as in Fig. 1D. Points and standard deviation (SD) indicate values for each individual mouse and SD, respectively. (B) Antigen-induced interferon γ (IFNγ)-secretions with freshly isolated CD8 T cells from pooled splenocytes in A were evaluated as in Fig. 1E. Results represent the average number of spots from duplicate wells with SD (bars) of the means. p-values were calculated using unpaired Student’s t test (ns, not significant; *p < 0.05, **p < 0.01, ***p < 0.001). (C) Therapeutic effectiveness of multi-epitope–loaded CD70-T_TriVax immunization against 3-day–established B16 melanoma. B6 mice (5 per group) were inoculated subcutaneously on day 0 with 1×105 B16 cells and were immunized i.v. on day 3 and 10 (vertical arrow) in combination with CD70-T or TriVax using either individual peptide or mixture of Trp1455/9M, Trp2180, and hgp10025 (3pepMIX) peptides as indicated. (D) Programmed death-1 blockade enhance the therapeutic efficacy of 3pepMIXCD70-T_TriVax immunization against advanced B16 tumors. B6 mice (7 per group) were inoculated subcutaneously (s.c.) on day 0 with 1×105 B16 cells and were immunized as described in Fig. 4B. Rat IgG (as controls) and anti–PD-L1 antibodies were administered intraperitoneally on days +1 and +3 after each immunization. Non-vaccinated mice (No vax) was included as a control. Tumor sizes were determined in individual mice by measurements of two opposing diameters and are presented as tumor areas on mm2. Points and bars indicate the mean for each group of mice and SD, respectively. p-values were calculated using 2-way ANOVA test (ns, not significant; *p < 0.05, ***p < 0.001). (E, F) In a parallel with D, mice (4 per group) were sacrificed on day 21, and the CD8 T-cell responses and immune cell populations were evaluated as described in Fig. 4C and D. (E) CD8 T-cell/Treg ratio as measured by percentage of CD8+ T cells per percentage of CD4+ Foxp3+ T cells cells in each group. Results represent the ave-rage percentage of the gated cells from individual mice with SD (bars) of the means. p-values were calculated using unpaired Student’s t test (*p < 0.05, **p < 0.01, ***p < 0.001). (F) The frequency of antigen-specific CD8 T cells of spleen and tumor site was evaluated by intracellular IFNγ staining. Points and bars indicate values for each individual mouse and SD, respectively. These experiments were repeated twice with similar results. |
6. Effects of CD70-T vaccination in antitumor efficacy of adoptive T-cell therapy
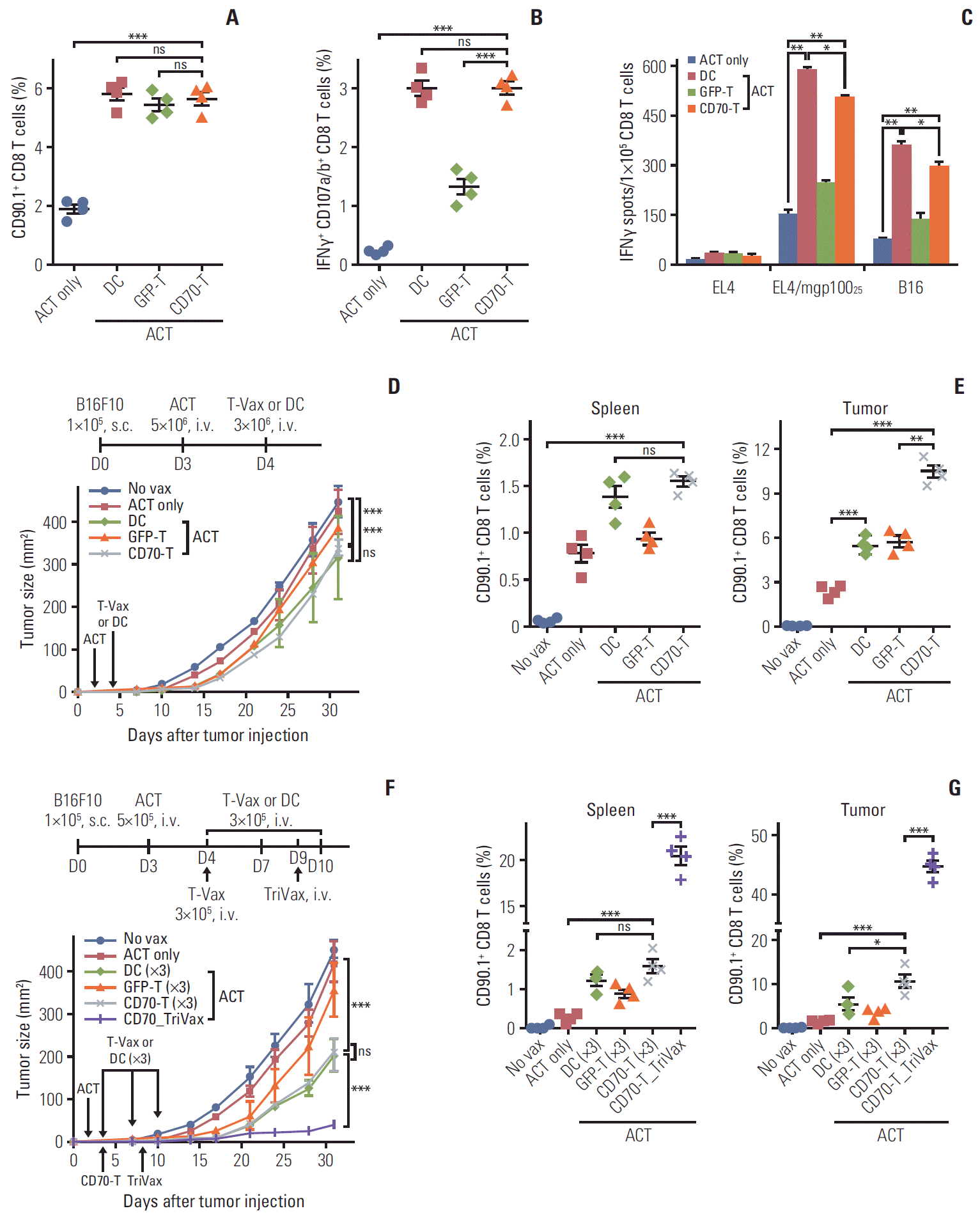 | Fig. 6.Effects of CD70-T vaccination in antitumor efficacy of adoptive T-cell therapy. (A-C) B6 mice (2 per group) were adoptively transferred 5×106 naïve Pmel-1 T cells on day 0, followed by immunization on day 1 with 3×106 hgp10025-pulsed GFP-T and CD70-T cells. Pmel-1 adoptive cell transfer (ACT) alone (ACT only) and dendritic cell vaccinated mice (DC) were included for comparison. (A) Seven days after the immunization, in vivo expansion of Pmel-1 T cells (CD90.1+CD8 T cells) in spleen was assessed. (B) The frequency of mgp10025-specific CD8 T cells in A was evaluated as in Fig. 1D. The results are the sum of two independent experiments with two per group. Points and bars indicate values for each individual mouse and SD, respectively. (C) Antigen-induced interferon γ (IFNγ)-secretions with freshly isolated CD8 T cells from pooled splenocytes in A were evaluated as in Fig. 1E. Results represent the average number of spots from duplicate wells with SD (bars) of the means. p-values were calculated using unpaired Student’s t test (ns, not significant; *p < 0.05, **p < 0.01, ***p < 0.001). (D, F) Antitumor effects of CD70-T immunization after Pmel-1 ACT. B6 mice (5 per group) were inoculated subcutaneously (s.c.) on day 0 with 1×105 B16 cells and received 5×106 Pmel-1 cells on day 3 followed by vaccination with hgp10025-pulsed GFP-T and CD70-T cells once (on day 4; D) or thrice (on day 4, 7, and 10; F, vertical arrows). For CD70-T_TriVax regimen, TriVax was administered intravenously (i.v.) 5 days after CD70-T vaccination. Non-vaccinated mice (No vax), Pmel-1 ACT alone (ACT only) and DC vaccinated mice (DC) were included for comparison. Tumor sizes were determined in individual mice by measurements of two opposing diameters and are presented as tumor areas in mm2. Points and bars indicate the mean for each group of mice and SD, respectively. p-values were calculated using 2-way ANOVA test (ns, not significant; ***p < 0.001). (E, G) In a parallel with D and F, respectively, mice (4 per group) were sacrificed on day 21, and the frequency of Pmel-1 T cells in spleen and tumor site was evaluated. Points and SD indicate values for each individual mouse and SD, respectively. p-values were calculated using unpaired Student’s t test (ns, not significant; *p < 0.05, **p < 0.01, ***p < 0.001). These experiments were repeated twice with similar results. |




 PDF
PDF Citation
Citation Print
Print


 XML Download
XML Download