Abstract
Myeloproliferative neoplasms (MPNs) are classified as chronic myeloid leukemia (CML) and Philadelphia chromosome-negative MPN. In MPN cases, the presence of a BCR-ABL1 translocation with a coexisting mutation is exceptionally rare. Herein, we report the first documented patient with CML harboring CALR mutation in Korea. A 33-year-old woman was referred to our hospital in February 2015 with splenomegaly, leukocytosis, and thrombocytosis. She was diagnosed with CML and started receiving nilotinib. In October 2015, a major molecular response was observed, but thrombocytosis persisted. A repeat bone marrow (BM) examination revealed no specific findings. However, as thrombocytosis worsened, we changed nilotinib to dasatinib. In May 2019, owing to persistent thrombocytosis, we repeated the BM examination and found CALR mutation (15.97%) on the MPN–next generation sequencing (NGS) test. We then retrospectively performed repeat MPN-NGS testing using the BM aspirate sample obtained in 2015 and found CALR mutation (10.64%).
Myeloproliferative neoplasms (MPNs) are a group of disorders in which bone marrow (BM) stem cells show abnormal growth and reproduction. In MPNs, abnormal stem cells produce excess numbers of one or more types of blood cells. These abnormal cells do not function properly and can cause serious health problems unless properly treated and controlled. Chronic myeloid leukemia (CML) is a type of MPN characterized by the translocation between chromosome 9 and 22, which is called Philadelphia chromosome, thereby resulting in the BCR-ABL1 oncogene. According to ‘The 2016 revision to the World Health Organization classification of myeloid neoplasm and acute leukemia’, other classical MPNs can be diagnosed after CML is excluded. However, recent cases with the coexistence of BCR-ABL1 and other MPN markers (i.e., CALR, JAK2, or MPL mutations) have been reported. Herein, we present a case of BCR-ABL1–positive CML with a CALR mutation in a patient with persistent thrombocytosis who maintained a major molecular response after treatment with a tyrosine kinase inhibitor (TKI).
A 33-year-old woman was referred to our hospital in February 2015 owing to an abdominal mass and abnormal laboratory values. Abdominal ultrasonography revealed splenomegaly (22 cm), and laboratory examinations revealed a white blood cell (WBC) count of 107,600/mm3 and a platelet (PLT) count of 906,000/mm3. BM examination revealed a marrow cellularity of 90%, with markedly increased megakaryocytes. Reticulin staining indicated grade 2-3 fibrosis. The karyotype was 45,XX,t(9;22)(q34;q11.2),der(13;14)(q10;q10)[15]. In case of der(13;14)(q10;q10), it was found in all cells continuously after treatment and presumed to be germline mutation. Fluorescence in situ hybridization analysis showed that 99.2% of 500 cells were positive for t(9;22), and real-time quantitative polymerase chain reaction showed that the transcript level for BCR-ABL1/ABL1IS (international scale [IS]) was 25.603%. JAK2 V617F was negative on conventional screening. Testing for CALR mutations was not yet available. Accordingly, the patient started receiving nilotinib. After 6 months of treatment with nilotinib, she achieved a major molecular response. In October 2015, she developed persistent thrombocytosis. A repeat BM examination did not show any specific findings. The karyotype was 46,XX,der(13;14) (q10;q10)[13]. However, as thrombocytosis worsened (PLT count, 1,006,000/mm3) and splenomegaly persisted, we changed nilotinib to dasatinib. We then added hydroxyurea as part of the treatment, after which the thrombocytosis was controlled slightly but it worsened subsequently (Figs. 1 and 2). In May 2019, follow-up BM examination was performed, and we used the BM aspiration sample for MPN–next-generation sequencing (NGS): the patient had CALR mutation (c.1099_1136delinsAGGT), with an allele frequency of 15.97%, and TET2 mutation (c.3409+1G>A), with an allele frequency of 7.83%. We then retrospectively performed repeat MPN-NGS testing using the BM aspiration sample obtained in 2015. We found that CALR mutation had been presence since 2015, with an allele frequency of 10.64%, but no TET2 mutation was observed. We reported a case of CALR-positive CML with persistent thrombocytosis during TKI treatment.
The study protocol was approved by the Institutional Review Board of the Soonchunhyang University Seoul Hospital (No. 2020-01-012). The study patient was informed as the purpose of the study and provided her consent.
CALR mutation was first recognized as a somatic mutation in patients with MPN who had no mutation in either JAK2 or MPL in 2013 [11]. CALR mutation is observed in approximately 70% of patients with essential thrombocythemia (ET) or primary myelofibrosis who did not carry a mutation in either JAK2or MPL[11,12]. CALR-mutant MPNs are characterized by a gene signature associated with activating JAK2 signaling, and CALR-mutants activate the JAK/STAT pathway through MPL, leading to excessive PLT production [13]. More than 50 different mutations in CALR have been described, but a 52 base-pair deletion (type 1) or a 5 base-pair insertion (type 2) account for more than 80% of mutations [13]. Pietra et al. [14] showed an association between CALR type 1 mutations and a significant increase in the risk of myelofibrotic transformation among ET cases, whereas type 2 muta-tions were preferentially associated with an ET phenotype, low risk of thrombosis despite a very high PLT count, and an indolent clinical course.
The presence of a BCR-ABL1 translocation with a coexisting mutation in CALRis exceptionally rare. A total of 10 cases have been reported since the first report was published in 2015, and the current report is the first in Korea (Table 1). In most cases, CALR mutation and BCR-ABL1 translocation were synchronously identified or BCR-ABL1 translocation was detected after CALR mutation. Otherwise, the presence of CALR mutation was not confirmed, but there was a case where the diagnosis was changed to CML on the basis of the findings of ET. To the best of our knowledge, there has been no report of CALR mutation after CML diagnosis. TKIs were ineffective for CALR-mutated clones, as shown in previous studies. Accordingly, physicians should be careful while changing the treatment of patients with CML who achieve a molecular response but do not obtain a hematologic response. Additional molecular mutations may be identified in patients with CML with the recent widespread use of NGS testing. A recent study showed the presence of molecular aberrations other than JAK2 V617F and BCR-ABL1 in 56% of patients with MPN, including mutations in TET2, ASXL1, RUNX1, CBL, DNMT3A, PHF6, SF3B1, and TP53[15]. In particular, TET2 is a putative tumor suppressor gene located on chromosome 4q24. Only a few studies revealed TET2 mutations in patients with MPN. However, there is no consensus regarding the exact role of TET2. In the current case, the relevance of identifying a new TET2 mutation during the course of the disease is unclear. Although patients with CML with BCR-ABL1 translocation have additional molecular aberrations, they are still diagnosed with CML on the basis of the current diagnostic criteria. However, further studies are needed regarding the diagnosis, treatment, and prognosis of these patients.
ACKNOWLEDGMENTS
This study was supported by the Soonchunhyang University Research Fund. We would like to thank Editage (www.editage.co.kr) for English language editing.
References
1. Cabagnols X, Cayuela JM, Vainchenker W. A CALR mutation preceding BCR-ABL1 in an atypical myeloproliferative neoplasm. N Engl J Med. 2015; 372:688–90.
2. Bonzheim I, Mankel B, Klapthor P, Schmidt J, Hinrichsen T, Wachter O, et al. CALR-mutated essential thrombocythemia evolving to chronic myeloid leukemia with coexistent CALR mutation and BCR-ABL translocation. Blood. 2015; 125:2309–11.

3. Loghavi S, Pemmaraju N, Kanagal-Shamanna R, Mehrotra M, Medeiros LJ, Luthra R, et al. Insights from response to tyrosine kinase inhibitor therapy in a rare myeloproliferative neoplasm with CALR mutation and BCR-ABL1. Blood. 2015; 125:3360–3.
4. Diamond JM, de Almeida AM, Belo HJ, da Costa MP, Cabecadas JM, Abecasis MM. CALR-mutated primary myelofibrosis evolving to chronic myeloid leukemia with both CALR mutation and BCR-ABL1 fusion gene. Ann Hematol. 2016; 95:2101–4.

5. Seghatoleslami M, Ketabchi N, Ordo A, Asl JM, Golchin N, Saki N. Coexistence of p190 BCR/ABL transcript and CALR 52-bp deletion in chronic myeloid leukemia blast crisis: a case report. Mediterr J Hematol Infect Dis. 2016; 8:e2016002.

6. Klairmont MM, Cheng J, Schwartzberg L, Ho HH, Gradowski JF. Chronic myeloid leukemia, BCR-ABL1-positive with CALR and MPL mutations. Int J Lab Hematol. 2018; 40:e41–2.
7. Dogliotti I, Fava C, Serra A, Gottardi E, Daraio F, Carnuccio F, et al. CALR-positive myeloproliferative disorder in a patient with Ph-positive chronic myeloid leukemia in durable treatment-free remission: a case report. Stem Cell Investig. 2017; 4:57.

8. Xia D, Hsi ED, Dal Cin P, Hasserjian RP. Composite chronic myeloid leukemia and essential thrombocythemia with BCRABL1 fusion and CALR mutation. Am J Hematol. 2019; 94:504–5.
9. Blouet A, Rousselet MC, Le Bris Y, Ribourtout B, Bouvier A, Cottin L, et al. Imatinib treatment of chronic myeloid leukemia reveals a preexisting CALR-mutated essential thrombocythemia. Hemasphere. 2018; 2:e29.

10. Lewandowski K, Gniot M, Wojtaszewska M, Kandula Z, Becht R, Paczkowska E, et al. Coexistence of JAK2 or CALR mutation is a rare but clinically important event in chronic myeloid leukemia patients treated with tyrosine kinase inhibitors. Int J Lab Hematol. 2018; 40:366–71.
11. Nangalia J, Massie CE, Baxter EJ, Nice FL, Gundem G, Wedge DC, et al. Somatic CALR mutations in myeloproliferative neoplasms with nonmutated JAK2. N Engl J Med. 2013; 369:2391–405.
12. Tefferi A, Lasho TL, Finke CM, Knudson RA, Ketterling R, Hanson CH, et al. CALR vs JAK2 vs MPL-mutated or triplenegative myelofibrosis: clinical, cytogenetic and molecular comparisons. Leukemia. 2014; 28:1472–7.

13. Cazzola M, Kralovics R. From Janus kinase 2 to calreticulin: the clinically relevant genomic landscape of myeloproliferative neoplasms. Blood. 2014; 123:3714–9.

14. Pietra D, Rumi E, Ferretti VV, Di Buduo CA, Milanesi C, Cavalloni C, et al. Differential clinical effects of different mutation subtypes in CALR-mutant myeloproliferative neoplasms. Leukemia. 2016; 30:431–8.

15. Martin-Cabrera P, Haferlach C, Kern W, Schnittger S, Haferlach T. BCR-ABL1-positive and JAK2 V617F-positive clones in 23 patients with both aberrations reveal biologic and clinical importance. Br J Haematol. 2017; 176:135–9.
Fig. 1.
White blood cell (WBC) and platelet counts during follow-up of a patient with chronic myeloid leukemia over the course of treatment.
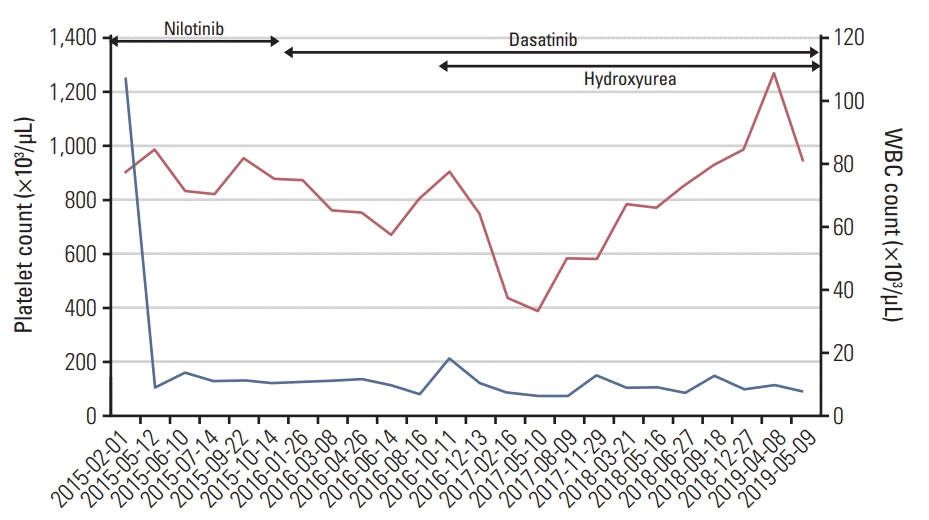
Fig. 2.
BCR-ABL1 transcript levels during follow-up of a patient with chronic myeloid leukemia over the course of treatment.
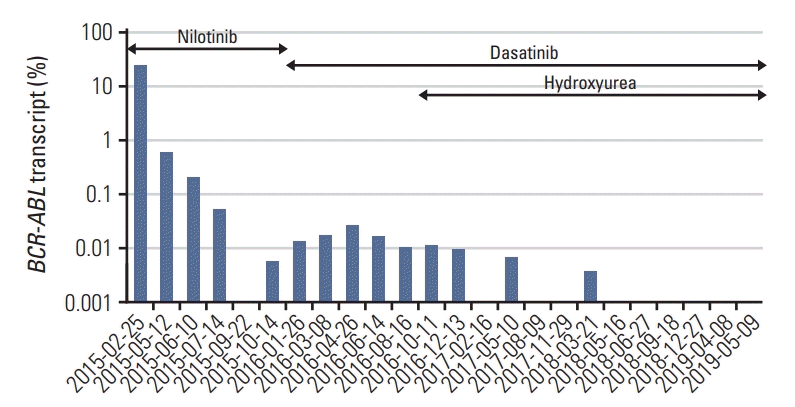
Table 1.
Cases with BCR-ABL1 translocation and CALR mutation
| Patient No. | Study | Age (yr)/Sex | Time of BCR-ABL1 detection | Type of CALR/Time of detection |
Treatment |
|
|---|---|---|---|---|---|---|
| Medication | Course | |||||
| 1 | Cabagnols et al. [1] | 73/F | At the time of diagnosis | Type 1/initial(R) | Ima → Dasa | Persistent thrombocytosis even though the BCR-ABL1 transcript level decreased |
| 2 | Bonzheim et al. [2] | 26/M | 3 yr after diagnosis (no mutation initially) | Type 2/initial(R) | IFN →Nilo | Persistent thrombocytosis and no change in the CALR allele burden even though the BCR-ABL1 transcript level decreased |
| 3 | Loghavi et al. [3] | 67/M | At the time of diagnosis | Type 1/initial(R) | Dasa | Sustained PMF findings on bone marrow biopsy even though the BCR-ABL1 transcript level decreased |
| 4 | Diamond et al. [4] | 50/M | At the time of diagnosis (no initial sample) | Type 1/1 yr later | Ima | Persistent anemia and splenomegaly even though the BCR-ABL1 transcript level decreased |
| 5 | Seghatoleslami et al. [5] | 78/F | At the time of diagnosis (p190 type) | Type 1/initial | No data | Coexistence of BCR-ABL1 transcript and CALR mutation in blast crisis in a patient with CML |
| 6 | Klairmont et al. [6] | 90/F | 4 yr after diagnosis (no mutation initially) | Type 1/11 yr later (no initial sample) | Ima | The initial presentation of thrombocytosis on a later bone marrow biopsy demonstrated a hypercellular marrow with megakaryocytic hyperplasia, reticulin fibrosis, and the absence of a BCR-ABL1 translocation. We therefore hypothesized that the patient initially harbored del52CALR (testing for which was not yet available at the time of diagnosis), leading to the clinical and histologic manifestations of a Ph-MPN. |
| The subsequent development of leukocytosis and acquisition of t(9;22)(q34;q11) suggests that CML evolved at a later point in time. | ||||||
| MPL V501A mutation was also identified. | ||||||
| 7 | Dogliotti et al. [7] | 61/F | At the time of diagnosis | Type 1/initial(R) | Ima | Persistent thrombocytosis even though the BCR-ABL1 transcript level decreased |
| 8 | Xia et al. [8] | 65/W | 4 yr after diagnosis (no mutation initially) | Type 1/4 yr later (no initial sample) | Dasa | The initial presentation of thrombocytosis with a bone marrow aspiration demonstrated a normocellular-appearing marrow with atypical large megakaryocytes, consistent with ET. The karyotype and testing for JAK2p.V617F and BCR-ABL1 showed normal results. |
| Mild leukocytosis and thrombocytosis were observed even though the BCR-ABL1 transcript level decreased | ||||||
| 9 | Blouet et al. [9] | 76/M | At the time of diagnosis | Type 1/initial(R) | Ima (+HU) | At diagnosis, the CALR mutation was present at a very low allele burden; it was not detectable on fragment analysis (sensitivity is ~5% of the allele burden) because of the higher representation of BCR-ABL1 clones. Tyrosine kinase inhibitor (TKI) treatment led to a decrease of the BCR-ABL1 clone and allowed the proliferation of the CALR clone that can explain the short time between the diagnosis of CML and ET. |
| 10 | Lewandowski et al. [10] | 55/F | At the time of diagnosis | Type 1/initial(R) | Ima→Nilo→Dasa | Persistent thrombocytosis even though the BCR-ABL1 transcript level decreased |
| HU/IFN | ||||||




 PDF
PDF Citation
Citation Print
Print


 XML Download
XML Download