INTRODUCTION
MATERIALS AND METHODS
Design
Patients
Therapeutic Management
Assessed Data
Definitions
Study Outcomes
Statistical Analysis
RESULTS
Baseline Characteristics and Proportion of AKI
Table 1.
| Variable | Total population (n=109) | AKI group (n=48) | No AKI group (n=61) | P-value |
|---|---|---|---|---|
| Age | 64 (57–71) | 69 (61–77) | 60 (55–66) | <0.001 |
| > 60 yr | 67 (61.5) | 37 (77) | 30 (49) | 0.003 |
| Sex (male:female) | 75:34 | 33:15 | 42:19 | 0.999 |
| BMI | 27 (24–31) | 27.3 (24.5–30.7) | 27 (24–32.5) | 0.620 |
| Obesity (BMI >30 kg/m2) | 32 (29.5) | 11 (23) | 21 (34.5) | 0.182 |
| Comorbidity | ||||
| Hypertension | 56 (51.5) | 30 (62.5) | 26 (42.6) | 0.054 |
| Prior use of ACE inhibitor/A2RB | 27 (25) | 16 (33.5) | 11 (18) | 0.071 |
| Diabetes | 44 (40) | 23 (48) | 21 (34.4) | 0.175 |
| Chronic renal failure | 5 (4.5) | 1 (2) | 4 (6.5) | 0.388 |
| Chronic respiratory failure | 20 (18.5) | 8 (16.5) | 12 (20) | 0.804 |
| Cardiomyopathy | 18 (16.5) | 9 (19) | 9 (15) | 0.613 |
| Immunocompromised | 6 (5.5) | 4 (8.3) | 2 (3.3) | 0.489 |
| Severity score | ||||
| APACHE II | 10.5 (7–16) | 13 (10–18) | 9 (6–11) | <0.001 |
| SAPS II | 30 (22–40) | 38 (31–53) | 24 (16–30.7) | <0.001 |
| SOFA | 4 (3–9) | 7 (4–11) | 4 (2–7.5) | 0.001 |
| Initial laboratory finding | ||||
| Baseline serum urea (g/L) | 0.57 (0.39–1.05) | 1 (0.71–1.3) | 0.45 (0.36–0.56) | <0.001 |
| Baseline creatinine (mg/L) | 9 (7–14) | 12.5 (9–18.4) | 8 (7–10) | <0.001 |
| Minimum P/F ratio | 66 (48–93) | 60 (46–78) | 73 (55–126) | 0.110 |
| WBC count (×109/L) | 12 (8.5–17) | 17 (7–21) | 10 (4–15) | 0.033 |
| Minimum lymphocyte (×103/µl) | 520 (312–720) | 510 (320–760) | 550 (302–715) | 0.819 |
| Platelet (×109/L) | 141 (48–238) | 134 (33–266) | 145 (58–229) | 0.772 |
| CRP (mg/L) | 199 (98–280) | 248 (171–326) | 151 (74–242) | 0.002 |
| Prothrombin time (%) | 60 (45–80) | 56 (41–69) | 75 (52–86) | 0.001 |
| Fibrinogen (g/L) | 5.1 (1.7–6.4) | 5.5 (4.2–6.9) | 2.5 (1.5–6.2) | 0.179 |
| D-dimer (µg/L) | 1,402 (653–3,881) | 2,222 (813–5,109) | 905 (598–1,757) | 0.002 |
| Lactate (mmol/L) | 2.1 (1.6–6) | 2.1 (1.7–4) | 1.9 (1.6–3.6) | 0.448 |
| CT scan lesion extension at admissiona, >50% | 33/78 (42) | 13/31 (42) | 20/47 (42.5) | 0.999 |
| Predisposing condition | ||||
| Sepsis | 49 (45) | 35 (73) | 14 (23) | <0.001 |
| ARDS | 81 (74) | 38 (81) | 43 (70.5) | 0.953 |
| ACPa | 10/66 (15) | 8/29 (27.5) | 2/37 (5.4) | 0.017 |
| Rhabdomyolysis | 21 (19) | 14 (29 | 7 (11.5) | 0.086 |
| Vasopressor | 60 (55) | 40 (84) | 20 (33) | <0.001 |
| Nephrotoxic drug | 29 (27) | 20 (42.5) | 9 (15) | 0.014 |
| Respiratory support | ||||
| Alternation HFNC/NIV | 61 (56) | 18 (37.5) | 43 (70.5) | - |
| MV | 48 (44) | 30 (62.5) | 18 (29.5) | 0.001 |
Values are presented as median (interquartile range) or number (%).
AKI: acute kidney injury; BMI: body mass index; ACE: angiotensin-converting enzyme; A2RB: angiotensin II receptor blockers; APACHE: Acute Physiology and Chronic Health Evaluation; SAPS: Simplified Acute Physiology Score; SOFA: Sequential Organ Failure Assessment; P/F ratio: ratio of the arterial partial pressure of oxygen and the inspiratory concentration of oxygen; WBC: white blood cell; CRP: C-reactive protein; CT: computed tomography; ARDS: acute respiratory distress syndrome; ACP: acute cor pulmonale; HFNC: high-flow nasal cannula; NIV: noninvasive ventilation; MV: mechanical ventilation.
AKI Characteristics and Risk Factors
Table 2.
| Variable | Odds ratio | P-value |
|---|---|---|
| Age > 60 yr | 1.35 | 0.320 |
| Hypertension | 1.58 | 0.612 |
| Prior use of ACE inhibitor/A2RB | 1.25 | 0.124 |
| Diabetes | 1.55 | 0.402 |
| P/F ratio <70 | 2.54 | 0.154 |
| CRP >200 mg/L | 2.21 | 0.140 |
| Fibrinogen >5 g/L | 3.03 | 0.115 |
| D-dimers >1,400 µg/L | 12.83 (1.9–85)a | 0.008 |
| Sepsis | 5.22 (0.94–28)a | 0.058 |
| Rhabdomyolysis | 6.53 | 0.072 |
| MV | 4.30 | 0.124 |
| Vasopressor | 1.82 | 0.466 |
| Nephrotoxic drug | 4.26 | 0.080 |
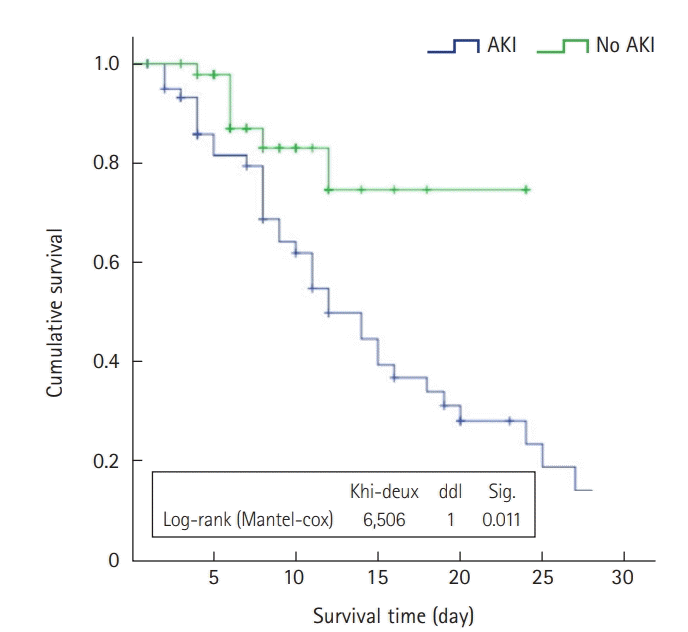
Outcomes
Table 3.
Values are presented as number (%) or median (interquartile range) unless otherwise indicated.
AKI: acute kidney injury; OR: odds ratio; CI: confidence interval; KDIGO: Kidney Disease: Improving Global Outcomes; NS: not significant; SOFA: Sequential Organ Failure Assessment; P/F ratio: the ratio of the arterial partial pressure of oxygen and the inspiratory concentration of oxygen; MV: mechanical ventilation.




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download